Physics
A radioactive nucleus X emits an alpha particle followed by two beta particles to form nucleus Y.
(a) With respect to the element X, where would you position the element Y in the periodic table?
(b) What is the general name of the elements X and Y?
(c) If the atomic number of Y is 80 then what is the atomic number of X?
Radioactivity
2 Likes
Answer
(a)
We can see from the above that X and Y have same atomic number. Hence, X and Y will occupy the same position in the periodic table
(b) Isotope.
(c) When the atomic number of Y is 80 and X and Y are isotopes then atomic number of X will also be 80.
Answered By
1 Like
Related Questions
The below picture shows a mother pushing her daughter sitting on a swing. The swing is going through the positions A, B, C where A and C are extreme positions and B is the mean position.
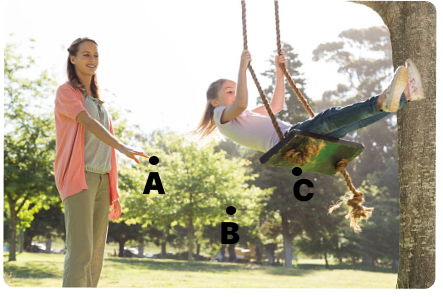
(a) Which is the right position i.e. at A, B or C, for the mother to give a constant periodic push to the swing every time in the forward direction to increase the amplitude of the swing?
(b) Name the phenomenon involved in this.
(c) Explain with this example how this phenomenon helps to increase the amplitude of the swing.
The circuit depicted in the figure is employed for studying Ohm's Law. Instead of using a standard resistor, a student opts for a glass tube filled with mercury (tube 1), connected to the circuit through two electrodes E1 & E2. He records the readings of the ammeter and voltmeter, thereby calculates the resistance. The student repeats the experiment by substituting tube 1 with tube 2, where the same amount of mercury fills the tube 2.
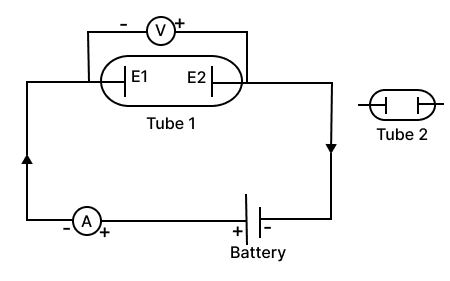
Neglecting internal resistance of the cell use (> or < or =) to compare
(a) the resistance in both the cases.
(b) the voltmeter readings in both the cases.
(c) the specific resistance in both the cases.
Observe the given circuit diagram and answer the questions that follow :
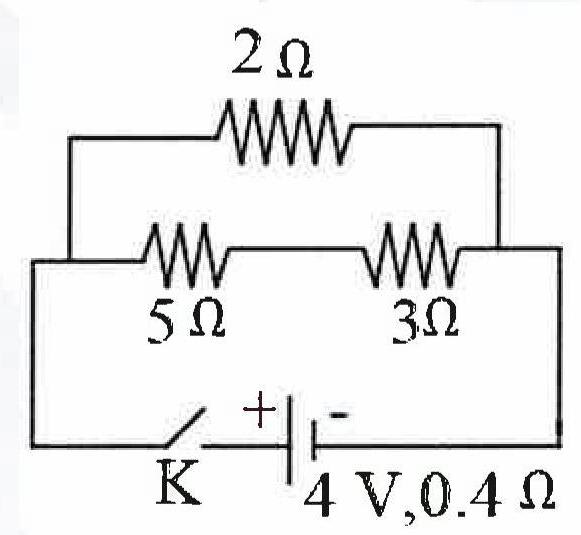
(a) Calculate the resistance of the circuit when the key K completes the circuit.
(b) Calculate the current through 3 Ω resistance when the circuit is complete.
What mass of ice at 0°C added to 2.1 kg water, will cool it down from 75°C to 25°C? Given Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹, Specific latent heat of ice = 336 J g⁻¹.