Chemistry
The salt which does not contain any water of crystallisation is :
P — Blue vitriol
Q — Gypsum
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Water
3 Likes
Answer
Only R
Reason — Water of crystallisation is the fixed number of water molecules chemically bound to a salt in its crystalline form. Salts like blue vitriol (CuSO4.5H2O) and gypsum (CaSO4.2H2O) are examples of hydrated salts. But baking soda (NaHCO3) doesn't have any water molecules in its formula. Therefore, it does not contain any water of crystallisation.
Answered By
2 Likes
Related Questions
The salt which is the cause of hardness in water:
- Sodium sulphate
- Magnesium bicarbonate
- Sodium chloride
- Calcium nitrate
Temporary hardness of water can be removed by:
- Adding sodium chloride
- Boiling
- Adding calcium carbonate
- Leaving it for a few hours.
Sodium chloride (common salt) besides being used in kitchen can also be used as the raw material for making:
P — Slaked lime
Q — Washing soda
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
The figure shown below demonstrates the solubility curve of a substance. From the statements given below, choose which is/are correct :
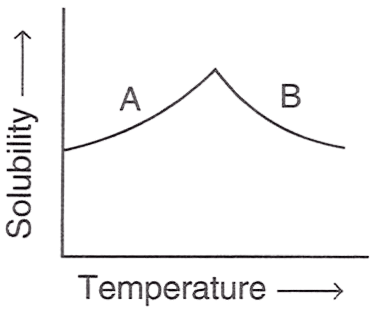
P — A is hydrated sodium chloride, B is anhydrous sodium chloride.
Q — A is Glauber's salt, B is sodium sulphate.
R — A is Gypsum, B is calcium sulphate.
- Only P
- Only Q
- Only R
- Both P and R