Chemistry
Sodium chloride (common salt) besides being used in kitchen can also be used as the raw material for making:
P — Slaked lime
Q — Washing soda
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Water
2 Likes
Answer
Both Q and R
Reason — Washing Soda (NaHCO3.10H2O) and baking Soda (NaHCO3) are made from sodium chloride using the Solvay process
NaCl + NH3 + CO2 + H2O ⟶ NaHCO3 + NH4Cl
NaHCO3 is filtered and heated to get sodium carbonate:
2NaHCO3 Na2CO3 + CO2 + H2O
Sodium carbonate (Na2CO3) is then hydrated to form washing soda.
Answered By
3 Likes
Related Questions
Temporary hardness of water can be removed by:
- Adding sodium chloride
- Boiling
- Adding calcium carbonate
- Leaving it for a few hours.
The salt which does not contain any water of crystallisation is :
P — Blue vitriol
Q — Gypsum
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
The figure shown below demonstrates the solubility curve of a substance. From the statements given below, choose which is/are correct :
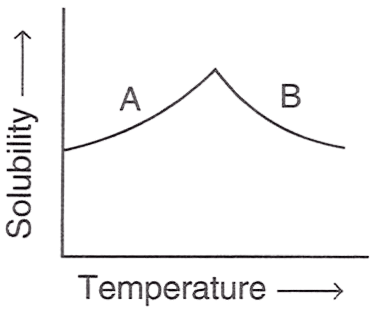
P — A is hydrated sodium chloride, B is anhydrous sodium chloride.
Q — A is Glauber's salt, B is sodium sulphate.
R — A is Gypsum, B is calcium sulphate.
- Only P
- Only Q
- Only R
- Both P and R
Assertion (A): Water is a universal solvent.
Reason (R): Water dissolves all substances except noble metals and glass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.