Physics
A spirit lamp supplying heat at a rate of 50 W is used to melt 0.025 kg of ice at 0 °C taken in a container. If all the ice in the container is melted in 168 s, then what is the specific latent heat of fusion of ice?
(The heat capacity of the container is negligible.)
Calorimetry
3 Likes
Answer
Given,
- Power of spirit lamp (P) = 50 W
- Mass of ice (m) = 0.025 kg
- Fixed temperature (Ti) = 0 °C
- Time Taken (t) = 168 s
Let specific latent heat of ice be Li.
Now,
Heat gained by ice (Q) = P x t = 50 x 168 = 8400 J
Also,
Li = = 336 x 103 J kg-1
Hence, specific latent heat of fusion of ice is 336 x 103 J kg-1.
Answered By
3 Likes
Related Questions
Study the diagram and answer the questions that follow:
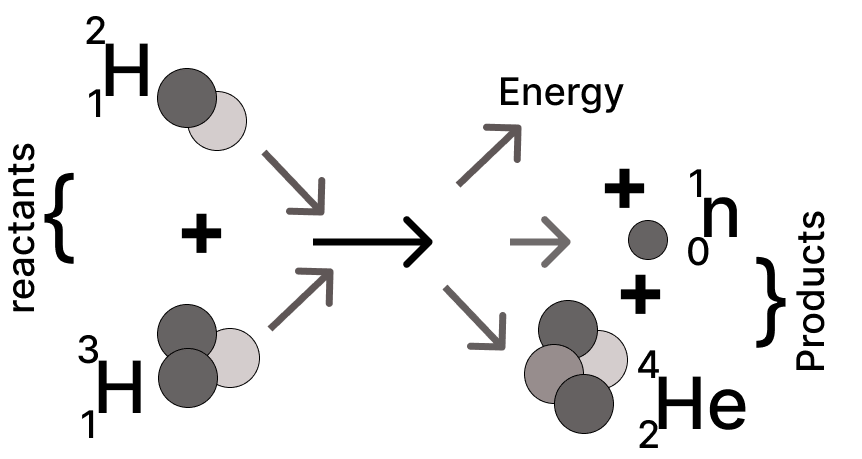
(a) Name the nuclear process displayed in the diagram.
(b) Is it possible to conduct this process at room temperature?
(c) Mass of reactants …………… mass of the products.
[Fill in the blank using <, > or =]
A cell of e.m.f 1.5 V and internal resistance 1 Ω is connected to two resistors of resistances 6 Ω and 3 Ω in parallel and a resistor of resistance 2 Ω in series as shown in the diagram.
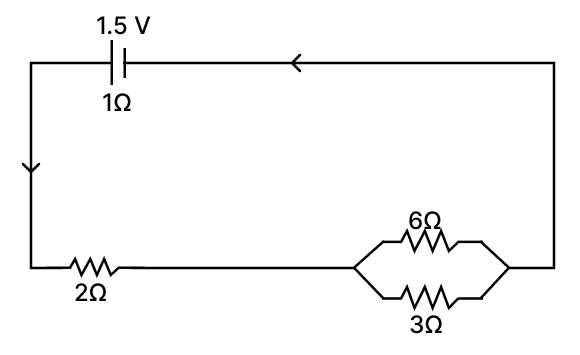
Calculate the current through:
(a) 2 Ω resistor
(b) 6 Ω resistor
(a) State the principle of calorimetry.
(b) Why should the surface of the calorimeter be polished?
(c) Why should the calorimeter be made of a material of low specific heat capacity?
A student wants to design a device to connect a bulb rated 10 W, 22 V, to the mains 220 V, so that the bulb operates at its rated voltage.
(a) Name the device he uses.
(b) State the principle involved in the working of this device.
(c) When the bulb is connected to the output of the device, calculate:
- Current drawn
- Resistance of the bulb