Chemistry
An atom is electrically neutral because :
P — Electrons, protons and neutrons are equal in number.
Q — Protons are equal to neutrons.
R — Protons are equal to electrons.
- Only P
- Only Q
- Only R
- Both Q and R
Related Questions
An atom is neutral, its nucleus contains:
P — Negatively charged particles.
Q — Neutral particles.
R — Positively charged particles.
- Only P
- Only Q
- Only R
- Both Q and R
Postulates of modern atomic theory are:
P — Atoms of an elements may not be alike.
Q — Atoms of elements combine in small whole numbers to form molecules.
R — Atoms can neither be created nor destroyed.
- Only P
- Only Q
- Only R
- Both P and Q
Select the correct statement(s) for the diagram shown below.
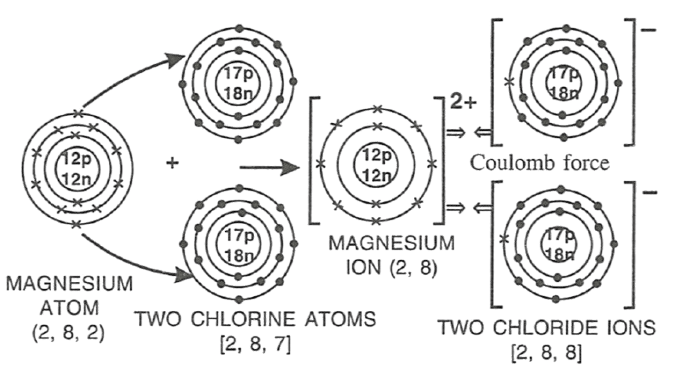
P — Mg looses electrons and undergoes reduction.
Q — Cl gains electrons and undergoes reduction.
R — Mg looses electrons and undergoes oxidation.
- Only P
- Only Q
- Only R
- Both Q and R
Select the correct statement(s) for the diagram shown below.
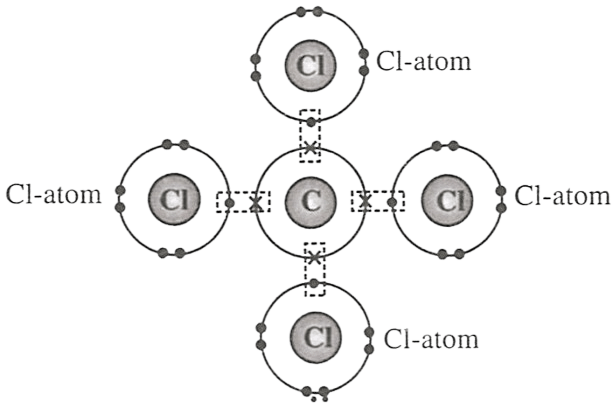
P — One atom of carbon transfers one electron to each chlorine atom.
Q — One atom of carbon shares four electron pairs, one with each of the four atoms of chlorine.
R — Carbon atom attains neon configuration and chlorine attains argon configuration after the combination.
- Only P
- Only Q
- Only R
- Both Q and R