Chemistry
Following figures illustrate graphical representations of gas laws. the figure which represents Charles' law is/are :
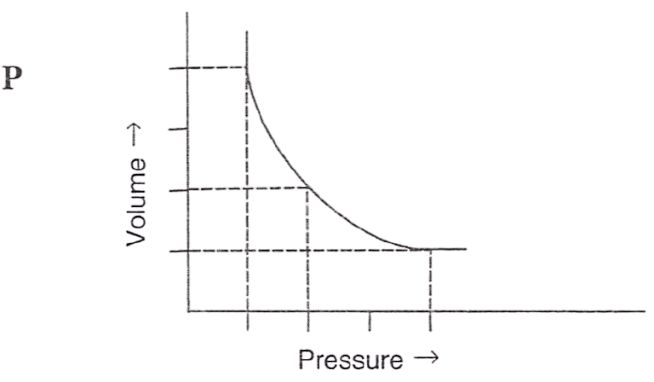
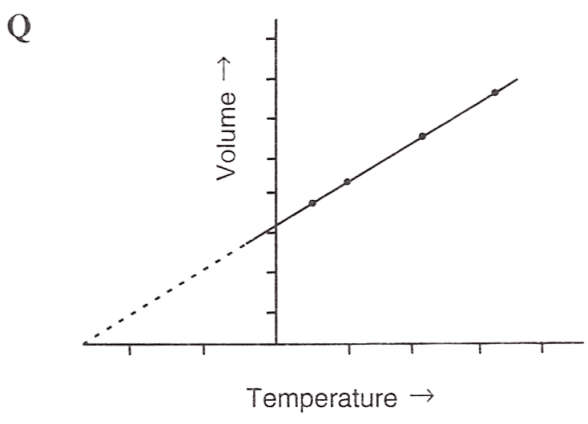
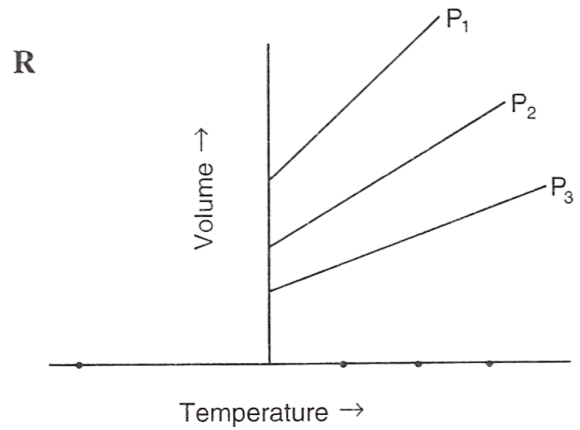
- Only P
- Only Q
- Only R
- Both Q and R
Related Questions
The volume of a given mass of a gas at constant temperature and 10 atmospheric pressure is 10 litres. Its volume at 5 atmospheres will be :
- 5 litres
- 10 litres
- 15 litres
- 20 litres
STP is called standard temperature and pressure. The standard temperature and standard pressure respectively are :
- 273 K and 760 mm
- 0°C and 760 cm
- 273°C and 1 atmosphere
- 373 K and 76 cm
The term used for the graph of volume vs pressure for the verification of Boyle's law is :
P — Isobar
Q — Isotherm
R — Isotope
- Only P
- Only Q
- Only R
- Both P and Q
Assertion (A): Inflating a balloon seems to violate Boyle's law.
Reason (R): Boyle's law is valid for a fixed mass. Here, the mass of gas is changing.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.