Chemistry
The action of heat on the blue crystalline solid X, gives a reddish brown gas Y, a gas which re-lights a glowing splint and leaves a black residue. When gas Z, which has a rotten egg smell, is passed through a solution of X, a black ppt. is formed.
(a) Identify X, Y and Z.
(b) Write equation for action of heat on X.
(c) Write equation between solution of X and gas Z.
Nitric Acid
74 Likes
Answer
(a) X is copper nitrate [Cu(NO3)2],
Y is nitrogen dioxide [NO2] and
Z is hydrogen sulphide [H2S].
(b) 2Cu(NO3)2 2CuO + 4NO2 + O2
(c) When a H2S gas, which has a rotten egg smell, is passed through a solution of Cu(NO3)2, a black ppt. of CuS is formed.
Cu(NO3)2 + H2S ⟶ CuS + 2HNO3
Answered By
38 Likes
Related Questions
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald's process. With respect to the process, answer the following questions :
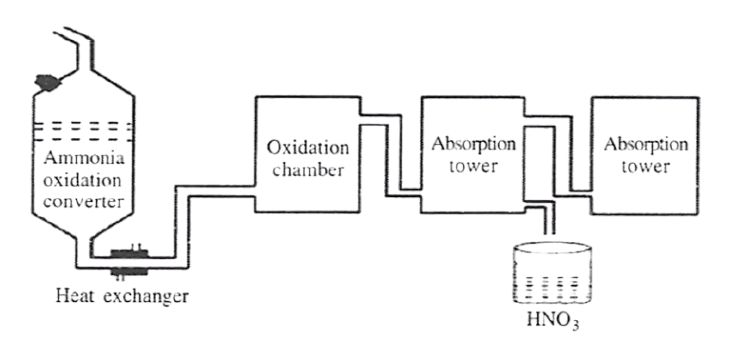
(a) Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
(b) Give balanced chemical equation for the reaction occurring during the conversion of nitrogen dioxide to nitric acid.
(a) Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
(b) Explain with the help of a balanced equation, the brown ring test for nitric acid.
(c) Why is freshly prepared ferrous sulphate solution used for testing the nitrate radical in the brown ring test
(a) Dilute nitric acid is generally considered a typical acid except for it's reaction with metals. In what way is dilute nitric acid different from other acids when it reacts with metals?
(b) Write the equation for the reaction of dilute nitric acid and conc. nitric acid with copper.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.

(a) Name A (a liquid), B (a solid) and C (a liquid). (Do not give the formulae).
(b) Write an equation to show how nitric acid undergoes decomposition.
(c) Write the equation for the reaction in which copper is oxidized by concentrated nitric acid.