Chemistry
(a) Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
(b) Explain with the help of a balanced equation, the brown ring test for nitric acid.
(c) Why is freshly prepared ferrous sulphate solution used for testing the nitrate radical in the brown ring test
Nitric Acid
54 Likes
Answer
(a)
1. To etch designs on copper and brassware.
Property : Acts as solvent for a large number of metals except noble metals
2. To purify gold.
Property : Gold may contain Cu, Ag, Zn, Pb etc. as impurities which dissolve in nitric acid.
3. To prepare aqua regia.
Property : It dissolves noble metals
(b) Brown ring test for nitric acid
To the aq. solution of a nitrate or nitric acid :
- Add freshly prepared saturated solution of iron [II] sulphate.
- Now add conc. sulphuric acid carefully from the sides of the test tube, so that it should not fall drop-wise in the test tube.
- Cool the test tube in water.
- A brown ring appears at the junction of the two liquids.
Equations for brown ring test:
6FeSO4 + 3H2SO4 + 2HNO3 (dil. ) ⟶ 3Fe2(SO4)3 + 4H2O +2NO
FeSO4 + NO ⟶ FeSO4.NO
[Nitroso Ferrous sulphate, a brown compound]
(c) A freshly prepared ferrous sulphate solution is used, because on exposure to the atmosphere, it is oxidised to ferric sulphate, which will not give the brown ring test.
Answered By
33 Likes
Related Questions
X, Y and Z are three crystalline solids which are soluble in water and have a common anion.
To help you to identify X, Y and Z, you are provided with the following experimental observations. Copy and complete the corresponding inferences in (a) to (e).(a) A reddish-brown gas is obtained when X, Y and Z are separately warmed with concentrated sulphuric acid and copper turning added to the mixture.
INFERENCE 1: The common anion is the …………… ion.
(b) When X is heated, it melts and gives off only one gas which re-lights a glowing splint.
INFERENCE 2: The cation in X is either …………… or ……………
(c) The action of heat on Y produces a reddish-brown gas and a yellow residue which fuses with the glass of the test tube.
INFERENCE 3: The metal ion present in Y is the …………… ion.
(d) When Z is heated, it leaves no residue. Warming Z with sodium hydroxide solution liberates a gas which turns moist red litmus paper blue.
INFERENCE 4: Z contains the …………… cation.
(e) Write the equations for the following reactions.
- X and concentrated sulphuric acid (below 200° C). (One equation only for either of the cations given in INFERENCE 2).
- Action of heat on Y.
- Concentrated nitric acid is added to copper turnings kept in a beaker.
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald's process. With respect to the process, answer the following questions :
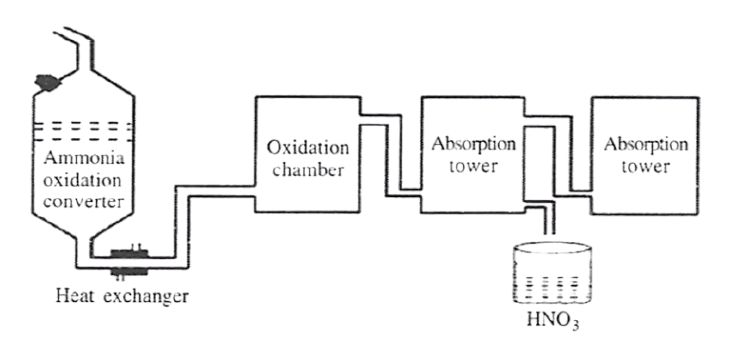
(a) Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
(b) Give balanced chemical equation for the reaction occurring during the conversion of nitrogen dioxide to nitric acid.
The action of heat on the blue crystalline solid X, gives a reddish brown gas Y, a gas which re-lights a glowing splint and leaves a black residue. When gas Z, which has a rotten egg smell, is passed through a solution of X, a black ppt. is formed.
(a) Identify X, Y and Z.
(b) Write equation for action of heat on X.
(c) Write equation between solution of X and gas Z.
(a) Dilute nitric acid is generally considered a typical acid except for it's reaction with metals. In what way is dilute nitric acid different from other acids when it reacts with metals?
(b) Write the equation for the reaction of dilute nitric acid and conc. nitric acid with copper.