Chemistry
The figure shown below demonstrates the solubility curve of a substance. From the statements given below, choose which is/are correct :
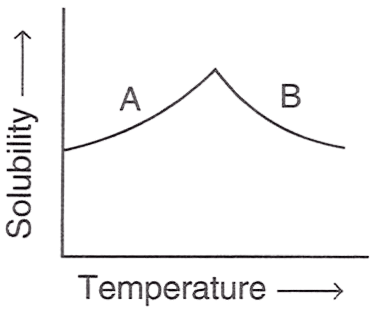
P — A is hydrated sodium chloride, B is anhydrous sodium chloride.
Q — A is Glauber's salt, B is sodium sulphate.
R — A is Gypsum, B is calcium sulphate.
- Only P
- Only Q
- Only R
- Both P and R
Answer
Only R
Reason — A is Glauber's salt and B is sodium sulphate because solubility curve of Na2SO4.10H2O (Glauber's salt) rises till it reaches 32.8°C, and then it falls slightly. This is because Na2SO4.10H2O is hydrous below 32.8°C and anhydrous above it.
Whereas solubility curve of sodium chloride and gypsum are almost flat, showing minimal change in solubility as temperature rises.
Related Questions
The salt which does not contain any water of crystallisation is :
P — Blue vitriol
Q — Gypsum
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Sodium chloride (common salt) besides being used in kitchen can also be used as the raw material for making:
P — Slaked lime
Q — Washing soda
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Assertion (A): Water is a universal solvent.
Reason (R): Water dissolves all substances except noble metals and glass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): A saturated solution becomes unsaturated on heating.
Reason (R): More amount of solute can dissolve in a solvent upon heating.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.