Physics
Two substances, A and B, have the same mass. Substance A has a higher specific heat capacity than substance B. What consequences would you expect when these substances are subjected to the same amount of heat energy?
- Substance A will experience a smaller temperature change than substance B.
- Substance A will experience a larger temperature change than substance B.
- Both substances will experience the same temperature change.
- Either of them can show a greater change in temperature as the temperature change depends on factors other than specific heat capacity.
Answer
Substance A will experience a smaller temperature change than substance B.
Reason — Specific heat capacity tells us how much heat is needed to raise the temperature of a substance. A higher specific heat means the substance absorbs more heat without a big temperature rise. So, with the same heat energy and mass, Substance A (with higher specific heat) will heat up less than Substance B.
Related Questions
The diagram shows the magnificent dome at Gol Ghumbaz at Bijapur. A boy standing at A whispers, and it is clearly heard at B across the huge dome.
[Speed of sound is 330 ms-1]
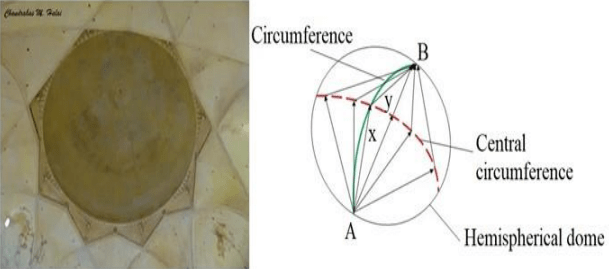
Assertion (A) : The distance travelled by sound waves (x+y) should be < 33 m.
Reason (R) : Sound reflected within 0.1 s is not heard by humans.
- A and R are true, and R is the reason for A.
- A and R are true, and R is not the reason for A.
- A is true, but R is false.
- A is false, but R is true.
A magnetic compass is present on a small table, very close to the wall in a room. A man enters the room and switches on the Air Conditioner fitted near the ceiling. He observes that the needle in the compass shows immediate deflection. Identify the most probable reason for the deflection.
- Air blowing in the room.
- Current starts flowing through AC wire concealed in the adjacent wall.
- Vibrations are produced when the Man starts talking to his wife in the kitchen.
- Vibrations were produced due to a file in his hand that fell on the ground.
A student traces the path of a ray of light through a glass prism for different angles of incidence. He analyses each diagram and draws the following conclusion:
I. On entering the prism, the light ray bends towards its base.
II. Light ray suffers refraction at the point of incidence and at the point of emergence while passing through the prism.
III. An emergent ray bends at a certain angle to the direction of the incident ray.
IV. While emerging from the prism at the second surface, the angle of refraction is less than the angle of incidence at that surface.
Which of the above inferences are correct?
- I, II and III
- I, III and IV
- II, III and IV
- All of these.
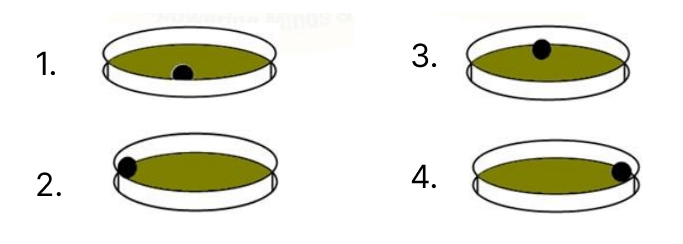
A metal ball is whirled inside a ring placed on a table, as shown in the diagram P. The diagram Q shows the motion of the ball after the ring is lifted.
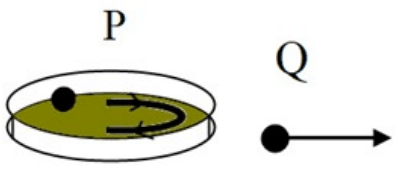
Which of the following diagrams shows the correct position of the metal ball in the ring when the ring is lifted?