Among the period 2 elements, the element which has the highest electron affinity is
- Lithium
- Carbon
- Chlorine
- Fluorine
Answer
Fluorine
Reason — Electronegativity increases as we move from left to right in a period, so element towards the end of the period has highest electronegativity. Hence, group 17 elements have highest electronegativity.
Among the following compounds identify the compound that has all three bonds (ionic, covalent and coordinate bond).
- Ammonia
- Ammonium chloride
- Sodium hydroxide
- Calcium chloride
Answer
Ammonium chloride
Reason — When ammonium chloride NH4Cl is formed cation NH4+ (having 3 covalent and one coordinate bond) and anion Cl- are attracted towards each other, due to electrical charge existing between them and ionic bond is formed. Thus, ammonium chloride is a good example of compound having all three types of bonds i.e., covalent, coordinate and ionic bond.
Identify the statement that is incorrect about alkanes.
- They are hydrocarbons.
- There is a single covalent bond between carbon and carbon.
- They can undergo both substitution as well as addition reactions.
- On complete combustion they produce carbon dioxide and water.
Answer
They can undergo both substitution as well as addition reactions.
Reason — The non availability of electrons in the single covalent bond makes them less reactive and therefore alkanes undergo characteristic substitution reactions only.
Which of these will act as a non-electrolyte?
- Liquid carbon tetrachloride
- Acetic acid
- Sodium hydroxide aqueous solution acid
- Potassium chloride aqueous solution.
Answer
Liquid carbon tetrachloride
Reason — It does not allow electric current to pass through it, neither in solution state nor in molten state.
Which one of the following will not produce an acid when made to react with water?
- Carbon monoxide
- Carbon dioxide
- Nitrogen dioxide
- Sulphur trioxide
Answer
Carbon monoxide
Reason — On reaction with water, CO2 produces carbonic acid, NO2 forms nitric acid and SO3 forms sulphuric acid whereas CO forms carbon dioxide gas.
CO + H2O ⟶ CO2 + H2
Identify the metallic oxide which is amphoteric in nature :
- Calcium oxide
- Barium oxide
- Zinc oxide
- Copper (II) oxide.
Answer
Zinc oxide
Reason — Zinc oxide is an amphoteric oxide. It reacts with both acids and alkalis giving salt and water.
In the given equation identify the role played by concentrated sulphuric acid.
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Oxidising agent
- Dehydrating agent
- None of the above
Answer
Oxidising agent
Reason — Oxidising property of concentrated sulphuric acid is due to the fact that on thermal decomposition, it yields nascent oxygen [O] which helps in oxidation.
H2SO4 ⟶ H2O + SO2 + [O]
Nascent oxygen oxidizes non-metals, metals and inorganic compounds.
Nitrogen gas can be obtained by heating
- Ammonium nitrate
- Ammonium nitrite
- Magnesium nitride
- Ammonium chloride
Answer
Ammonium nitrite
Reason — NH4NO2 2H2O + N2
Which of the following is not a typical property of an ionic compound?
- High melting point.
- Conducts electricity in the molten and in the aqueous solution state.
- They are insoluble in water.
- They exist as oppositely charged ions even in the solid state.
Answer
Are insoluble in water
Reason — Ionic compounds are soluble in water. They are insoluble in organic solvents. Water [polar solvent] has a high dielectric constant i.e. capacity to weaken the force of attraction, thus resulting in free ions.
The metals zinc and tin are present in the alloy :
- Solder
- Brass
- Bronze
- Duralumin.
Answer
Bronze
Reason — Bronze — Cu [80%], Zn [1%], Sn [19%]
Give one word or phrase for the following statements :
(i) A bond formed by a shared pair of electrons with both electrons coming from the same atom.
(ii) A salt formed by incomplete neutralisation of an acid by a base.
(iii) A reaction in which hydrogen of an alkane is replaced by a halogen.
(iv) A definite number of water molecules bound to some salts.
(v) The process in which a substance absorbs moisture from the atmospheric air to become moist, and ultimately dissolves in the absorbed water.
Answer
(i) Coordinate bond
(ii) Acid salt
(iii) Halogenation reaction
(iv) Water of crystallization
(v) Deliquescence
Write a balanced chemical equation for each of the following
(i) Action of ammonia with excess of chlorine.
(ii) Ammonia burns in oxygen.
(iii) Sulphur reacts with conc. nitric acid.
(iv) Calcium hydrogen carbonate with HCl.
(v) Carbon with conc. H2SO4.
Answer
(i) 8NH3 [excess] + 3Cl2 ⟶ 6NH4Cl + N2
(ii) 4NH3 + 3O2 ⟶ 2N2 + 6H2O
(iii) S + 6HNO3 ⟶ H2SO4 + 2H2O + 6NO2
(iv) Ca(HCO3)2 + 2HCl ⟶ CaCl2 + 2H2O + 2CO2
(v) C + 2H2SO4 (conc.) ⟶ CO2 + 2SO2 + 2H2O
Give the structural formula of the following
(i) diethyl ether
(ii) 1-propanal
(iii) acetone
(iv) 1, 2, dichloroethane
(v) ethanoic acid
Answer
(i) Diethyl ether

(ii) 1-propanal

(iii) Propanone [acetone]

(iv) 1, 2, dichloroethane

(v) ethanoic acid

State one appropriate observation for each of the following:
(i) Concentrated sulphuric acid is added drop wise to a crystal of hydrated copper sulphate.
(ii) Copper sulphide is treated with dilute hydrochloric acid.
(iii) Excess of chlorine gas is reacted with ammonia gas.
(iv) A few drops of dilute hydrochloric acid are added to silver nitrate solution, followed by addition of ammonium hydroxide solution.
(v) Electricity is passed through molten lead bromide.
Answer
(i) When concentrated Sulphuric acid is added dropwise to the crystals of hydrated copper sulphate, it changes the colour of hydrated copper Sulphate from blue to white.
(ii) A colourless gas having smell of rotten eggs is given off. The gas evolved is H2S
CuS + 2HCl ⟶ CuCl2 + H2S
(iii) Colourless ammonia gas reacts with greenish yellow excess chlorine giving a yellow explosive liquid (Nitrogen trichloride).
NH3 + 3Cl2 [excess] ⟶ 3HCl + NCl3
(iv) Curdy white precipitate of silver chloride [AgCl] is obtained.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(v) At cathode, a silvery grey deposit of lead is observed due to the discharge of lead ions (Pb2+) as neutral lead atoms.
Name the gas that is produced in each of the following cases:
(i) sodium propionate is heated with soda lime.
(ii) potassium sulphite is treated with dilute hydrochloric acid.
(iii) Sulphur is treated with concentrated nitric acid.
(iv) a few crystals of KNO3 are heated in a hard glass test tube.
(v) concentrated hydrochloric acid is made to react with manganese dioxide.
Answer
(i) Ethene gas [C2H4]
(ii) Sulphur dioxide gas [SO2]
(iii) Nitrogen dioxide gas [NO2]
(iv) Oxygen gas [O2]
(v) Chlorine gas [Cl2]
From the list given below, select the word(s) required to correctly complete blanks (i) to (v) in the following passage. The words from the list are to be used only once. Write the answers as (a) (i), (ii), (iii) and so on. Do not copy the passage.
[ammonia, ammonium, carbonate, carbon dioxide, hydrogen, hydronium, hydroxide, precipitate, salt, water]:
(i) A solution M turns blue litmus red, so it must contain (i) ............... ions; another solution O turns red litmus blue and hence, must contain (ii) ............... ions.
(ii) When solution M and O are mixed together, the products will be (iii) ...............and (iv) ...............
(iii) If a piece of magnesium was put into a solution M, (v) ............... gas would be evolved.
Answer
(i) A solution M turns blue litmus red, so it must contain (i) hydronium ions; another solution O turns red litmus blue and hence, must contain (ii) hydroxide ions.
(ii) When solution M and O are mixed together, the products will be (iii) salt and (iv) water
(iii) If a piece of magnesium was put into a solution M, (v) hydrogen gas would be evolved.
An element Z has atomic number 16. Answer the following questions on Z:
(i) State the period and group to which Z belongs.
(ii) Is Z a metal or a non-metal?
(iii) State the formula between Z and Hydrogen.
(iv) What kind of a compound is this?
Answer
(i) The element belongs to 3rd period and 16 [VI A] group.
Reason — The element Z has atomic number 16 and so the electronic configuration will be 2, 8, 6. The number of shells present in an atom determines it's period. Hence, Z will belong to 3rd period as it has three shells. The number of valence electrons determine the group of the element. Hence, Z will belong to group 16 [VI A] as it has 6 electrons in the valence shell.
(ii) Z is a non-metal.
(iii) It forms H2Z
(iv) It is a weak acid.
M is a metal above hydrogen in the activity series and its oxide has the formula M2O. This oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity. In the above context answer the following :
(i) What kind of combination (bond) exists between M and O?
(ii) How many electrons are there in the outermost shell of M ?
(iii) State the reaction taking place at the cathode.
(iv) Which electrode: anode or cathode is the oxidising electrode? Why?
Answer
(i) Electrovalent bond exits between M and O because the bond is formed between a metal and non-metal due to oppositely charged ions.
(ii) Number of electrons in the outer most shell of M is 1. It is so because the valency of O is -2 and as 2 atoms of M combine with O to form M2O, hence we can say that M has 1 valence electron.
(iii) At cathode : M+ (aq.) + e- ⟶ M (s)
(iv) Anode is the oxidizing electrode.
Reason — The anions donate the excess electrons to the anode and are oxidized to neutral atoms. Hence, the anode is the oxidizing electrode by which the electrons leave the electrolyte
Name the kind of particles present in : Carbonic acid.
Answer
Carbonic acid is a weak acid hence, it contains ions (H+, HCO3, CO32- ) and molecules (H2CO3)
Name the following :
(i) The property possessed by metals by which they can be beaten into sheets.
(ii) A compound added to lower the fusion temperature of electrolytic bath in the extraction of aluminium.
(iii) The ore of zinc containing its sulphide.
Answer
(i) Malleability
(ii) Cryolite
(iii) Zinc Blende [ZnS]
Give one equation each to show the following properties of sulphuric acid:
(i) Dehydrating property
(ii) Acidic nature
(iii) As a non-volatile acid
Answer
(i)
(ii) Na2SO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + SO2
(iii)
Give balanced chemical equations to prepare the following salts :
(i) Lead sulphate from lead carbonate.
(ii) Sodium sulphate using dilute sulphuric acid
(iii) Copper chloride using copper carbonate.
Answer
(i) Lead sulphate from lead carbonate:
PbCO3 + 2HNO3 ⟶ Pb(NO3)2 + H2O + CO2
Pb(NO3)2 + Na2SO4 ⟶ PbSO4 + 2NaNO3
(ii) Sodium sulphate using dilute sulphuric acid:
Na2CO3 + H2SO4 ⟶ Na2SO4 + H2O + CO2
(iii) Copper chloride using copper carbonate:
CuCO3 + 2HCl (dil) ⟶ CuCl2 + H2O + CO2
Solve the following:
(i) What volume of oxygen is required to burn completely 90 dm3 of butane under similar conditions of temperature and pressure?
2C4H10 + 13O2 ⟶ 8CO2 + 10H2O
(ii) The vapour density of a gas is 8. What would be the volume occupied by 24.0 g of the gas at STP?
(iii) A vessel contains X number of molecules of hydrogen gas at a certain temperature and pressure. How many molecules of nitrogen gas would be present in the same vessel under the same conditions of temperature and pressure?
Answer
(i) [By Lussac's law]
To calculate the volume of oxygen
∴
Hence, volume of oxygen required is 585 dm3
(ii) Given, V.D. = 8
Gram molecular mass = V.D. x 2 = 8 x 2 = 16 g.
16 g occupies 22.4 lit.
∴ 24 g. will occupy = x 24 = 33.6 lit.
Hence, volume occupied by gas = 33.6 lit.
(iii) According to Avogadro's law, equal volume of all gases under similar conditions of temperature and pressure contain equal number of molecules.
Hence, number of molecules of N2 = Number of molecules of H2 = X
Define:
- Mole
- Gay Lussac's law
(ii) A cylinder contains 68 g of ammonia gas at s.t.p.
(1) What is the volume occupied by this gas ?
(2) How many moles of ammonia are present in the cylinder ?
(3) How many molecules of ammonia are present in the cylinder ?
[N—14, H—1]
Answer
(i) Mole — A mole is the amount of substance which contains the same number of units as the number of atoms in 12,000 g of carbon - 12 [6C12]
(ii) Gay Lussac's law — When gases react, they do so in volumes which bear a simple ratio to one another, and to the volume of the gaseous product, provided that all the volumes are measured at the same temperature and pressure.
(ii) (1) Gram molecular mass of ammonia = N + 3(H) = 14 + 3 = 17 g.
17 g. occupies 22.4 lit. of vol.
∴ 68 g will occupy = x 68 = 89.6 lit.
Hence, volume occupied by this gas = 89.6 lit.
(2) 17 g = 1 mole
∴ 68 g = x 68 = 4 moles.
(3) 1 mole = 6 × 1023
∴ 4 moles = 4 x 6.023 × 1023 molecules.
Hence, Moles = 4 and molecules = 24.092 × 1023 molecules
Write balanced equation for the following reactions to take place:
(i) Catalytic hydrogenation of ethyne.
(ii) Preparation of ethyne from ethylene dibromide.
(iii) Preparation of ester using carboxylic acid.
(iv) Reaction of sodium with ethanol.
(v) Preparation of ethene using alkylhalide
Answer
(i) Catalytic hydrogenation of ethyne.
(ii) Preparation of ethyne from ethylene dibromide.
(iii) Preparation of ester using carboxylic acid.
(iv) Reaction of sodium with ethanol
(v) Preparation of ethene using alkylhalide
Aluminium is obtained from alumina:
Answer the following :
(i) Electrolyte used
(ii) Write cathode reaction
(iii) Why anode is replaced
Answer
(i) Mixture of fused alumina (Al2O3), cryolite (Na3AlF6) and fluorspar [CaF2]
(ii) At cathode : 2Al3+ + 6e- ⟶ 2Al
(iii) The anodes are continuously replaced during the electrolysis because :
- The oxygen evolved at the anode escapes as a gas or reacts with the carbon anode.
- The carbon anode is thus oxidized to carbon monoxide which either burns giving carbon dioxide or escapes out through an outlet.
2C + O2 ⟶ 2CO [2CO + O2 ⟶ 2CO2] - The carbon anode is hence consumed and renewed periodically after a certain period of usage.
Give balanced equations for the following:
(i) Laboratory preparation of nitric acid.
(ii) Preparation of ethanol from monochloroethane and aq. sodium hydroxide.
Answer
(i) Laboratory preparation of nitric acid
KNO3 [conc.] + H2SO4 [conc.] KHSO4 + HNO3
(ii) Preparation of ethanol from monochloroethane and aq. sodium hydroxide.
State your observation in each of the following cases :
(i) When dilute hydrochloric acid is added to sodium carbonate crystals.
(ii) When excess sodium hydroxide is added to calcium nitrate solution.
(iii) At the cathode when acidified aqueous copper sulphate solution is electrolyzed with copper electrodes.
(iv) When calcium hydroxide is heated with ammonium chloride crystals.
(v) When moist starch iodide paper is introduced into chlorine gas.
Answer
(i) Effervescence of a gas are seen which turns lime water milky confirming that the gas is CO2.
Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2 ↑
(ii) Milky white precipitate is obtained which is sparingly soluble in excess of NaOH.
Ca(NO3)2 + 2NaOH ⟶ Ca(OH)2 ↓ + 2NaNO3
(iii) Copper, a brownish pink metal is deposited at the cathode when acidified aq. CuSO4 soln. is electrolysed with copper electrodes.
(iv) Pungent smelling gas (ammonia) is given out.
2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3
(v) Chlorine gas turns moist starch iodide paper blue black.
Cl2 + 2KI ⟶ 2KCl + I2
[Starch + I2 ⟶ blue black colour]
Study the figure given below and answer the questions that follow :

(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate ?
(iii) Name another gas which has the same property and can be demonstrated through the experiment.
Answer
(i) Dry HCl (Hydrogen chloride) gas is the gas Y
(ii) High solubility of HCl in water
(iii) Ammonia (NH3) gas also demonstrates high solubility in water.
(i) Name the other ion formed when ammonia dissolves in water.
(ii) Give one test that can be used to detect the presence of the ion produced.
Answer
(i) Hydroxyl ion [OH-1] and ammonium ions [NH4+] are formed.
NH3 + H2O ⟶ NH4OH
NH4OH ⇌ NH4+ + OH-1
(ii) The alkaline NH4OH, due to the presence of hydroxyl ions [OH-] turns red litmus blue and phenolphthalein soln. pink
Give a chemical test to distinguish between the following pairs of compounds:
(i) Sodium chloride solution and sodium nitrate solution.
(ii) Hydrogen chloride gas and hydrogen sulphide gas.
(iii) Ethene gas and ethyne gas.
(iv) Calcium nitrate solution and zinc nitrate solution.
(v) Potassium carbonate and potassium sulphite.
Answer
(i) Add silver nitrate soln. to the given solns., sodium chloride reacts to form a white ppt. which is soluble in NH4OH and insoluble in dil. HNO3. The other soln. is sodium nitrate.
NaCl + AgNO3 ⟶ AgCl ↓ [white ppt.] + NaNO3
NaNO3 + AgNO3 ⟶ no white ppt.
(ii) Hydrogen sulphide gas turns moist lead acetate paper silvery black or black whereas, no change is observed in case of HCl gas.
Pb(CH3COO)2 [colourless] + H2S ⟶ PbS [black] + 2CH3COOH
(iii) When ammoniacal silver nitrate is added to the two solutions ethyne forms a white ppt. of silver acetylide, whereas, no change appears in ethene.
(iv) When NaOH is added to the given soln., Zinc nitrate, reacts to form a gelatinous white ppt. which dissolves in excess of NaOH whereas, Calcium nitrate forms a milky white ppt. which is insoluble in excess of NaOH. Hence, the two can be distinguished
(v) When dil. sulphuric acid is added to potassium carbonate and heated, colourless, odourless gas is evolved which turns lime water milky and has no effect on KMnO4 or K2Cr2O7 solutions.
When dil. sulphuric acid is added to potassium sulphite and heated, colourless gas with suffocating odour is evolved which turns lime water milky. It turns acidified K2Cr2O7 from orange to clear green and pink coloured KMnO4 to clear colourless.
Hence, the two compounds can be distinguished.
Draw the structure of the stable positive ion formed when an acid dissolves in water.
Answer
Hydronium ion is the stable positive ion formed when an acid dissolves in water. Its structure is shown below:
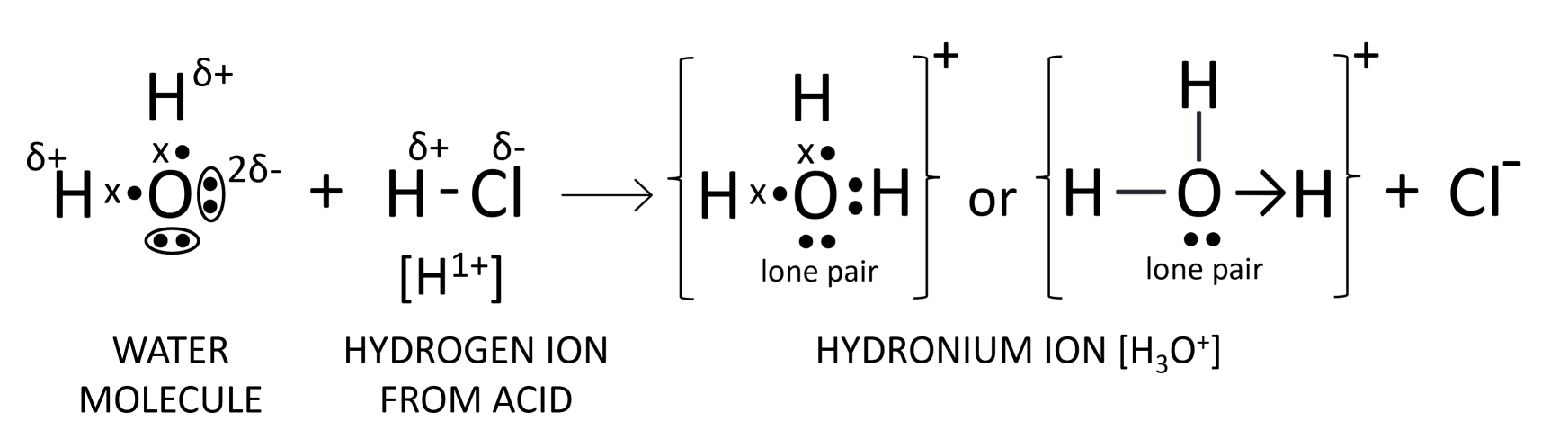
State the inference drawn from the following observations :
(i) On carrying out the flame test with a salt P a brick red flame was obtained. What is the cation in P ?
(ii) A gas Q turns moist lead acetate paper silvery black. Identify the gas Q.
(iii) pH of liquid R is 10. What kind of substance is R?
Answer
(i) Cation in P is Ca2+ (calcium ion)
(ii) The gas Q is H2S (Hydrogen sulphide).
Pb(CH3COO)2 [colourless] + H2S ⟶ PbS [black] + 2CH2COOH
(iii) R is an alkaline substance.