The symbol of Beryllium is :
- B
- Ba
- Be
- Br
Answer
Be
Reason — The symbol of Beryllium is Be.
Which of the following is a pair of metalloids ?
- Na and Mg
- B and Si
- C and P
- He and Ar
Answer
B and Si
Reason — Na (Sodium) and Mg (Magnesium) are both metals. B (Boron) and Si (Silicon) are both metalloids. C (Carbon) and P (Phosphorus) are both nonmetals. He (Helium) and Ar (Argon) belong to the noble gas group.
Which of the following properties is not shown by compounds ?
- They are heterogeneous.
- They are homogeneous.
- They have definite molecular formulae.
- They have fixed melting and boiling points.
Answer
They are heterogeneous.
Reason — Compounds are homogeneous, meaning their components are uniformly mixed at the molecular level.
Which of the following elements has atomicity 4?
- Iodine
- Ozone
- Phosphorus
- Sulphur
Answer
Phosphorus
Reason — Phosphorus has atomicity 4.
A molecule is the smallest unit of :
- an element
- a compound
- a mixture
- an ion
Answer
a compound
Reason — A molecule is the smallest unit of a compound that retains all the chemical properties of that compound.
Which of the following substances dissolves in carbon disulphide ?
- Iron
- Sulphur
- Sodium chloride
- Iron sulphide
Answer
Sulphur
Reason — Sulphur is soluble in carbon disulphide.
Name:
(a) a soft metal
(b) a metal which is brittle
(c) a non-metal which is lustrous
(d) a liquid metal
(e) a metal which is a poor conductor of electricity
(f) a non-metal which is a good conductor of electricity
(g) a liquid non-metal
(h) the hardest naturally occurring substance
(i) an inert gas
Answer
(a) Sodium
(b) Zinc
(c) Graphite
(d) Mercury
(e) Tungsten
(f) Graphite
(g) Bromine
(h) Diamond
(i) Neon
Give two examples for each of the following:
(a) Metals
(b) Non-metals
(c) Metalloids
(d) Inert gases
Answer
(a) Gold and Silver
(b) Oxygen and Sulphur
(c) Boron and Silicon
(d) Helium and Neon
Write the chemical names of the following and also give their molecular formulae:
(a) Baking soda
(b) Vinegar
(c) Marble
(d) Sand
Answer
| S. No. | Common Name | Chemical Name | Molecular Formula |
|---|---|---|---|
| (a) | Baking soda | Sodium bicarbonate | NaHCO3 |
| (b) | Vinegar | Acetic acid | CH3COOH |
| (c) | Marble | Calcium carbonate | CaCO3 |
| (d) | Sand | Silicon dioxide | SiO2 |
Define:
(a) Elements
(b) Compounds
Answer
(a) An element is a pure substance which cannot be converted into anything simpler than itself by any physical or chemical process. It is made up of only one kind of atoms.
(b) A compound is a pure substance composed of two or more elements combined chemically in a definite proportion by mass.
Why is iron sulphide a compound?
Answer
Iron sulphide is a compound because of the following reasons:
- Iron sulphide is a compound formed when iron and sulphur combine chemically, on heating, in 7 : 4 ratio by mass.
- Iron is a grey black metal and Sulphur is a yellow amorphous non-metallic solid whereas iron sulphide is a black solid.
- Iron is attracted by a magnet and Sulphur is soluble in carbon disulphide but iron sulphide is neither attracted by a magnet nor it is soluble in carbon disulphide.
From the above, we can say that properties of iron sulphide are entirely different from its constituent elements i.e., iron and sulphur. Hence, iron sulphide is a compound.
Differentiate between:
(a) Pure and impure substances
(b) Homogeneous and heterogeneous substances
Answer
(a) Difference between Pure and impure substances:
| Pure substance | Impure substance |
|---|---|
| Pure substances have a definite chemical composition and definite physical and chemical properties. | Impure substances are made up of two or more pure substances mixed together in any proportion. They do not have any definite set of properties. |
| Pure substances are all homogeneous, i.e. their composition is uniform throughout the bulk. | Impure substances may be homogeneous or heterogeneous i.e. their composition is not uniform throughout the bulk. |
| Examples are gold, silver, water, sodium chloride etc. | Examples are air, sea water, solution of sugar in water etc. |
(b) Difference between Homogeneous and heterogeneous substances:
| Homogeneous substance | Heterogeneous substance |
|---|---|
| In Homogeneous substance, the components are uniformly distributed throughout its volume and cannot be seen separately. | In Heterogeneous substance, the components are not uniformly distributed throughout its volume and can be easily seen separately. |
| Examples are tap water, milk, brass, bronze etc. | Examples are soil, mud and water, sand and stone etc. |
How is sodium chloride different from its constituent elements? Explain.
Answer
The properties of sodium chloride are completely different from those of sodium and chlorine. Sodium is a soft, highly reactive metal. Chlorine is a poisonous greenish yellow gas with choking smell. But sodium chloride is a white crystalline solid which is non-poisonous and useful. It is added to our food as a mineral and also to add taste to it.
State four differences between elements and compounds.
Answer
Four differences between elements and compounds are:
| Elements | Compounds | |
|---|---|---|
| 1. | An element is the simplest type of a pure substance. | A compound is a pure substance, made up of two or more elements combined chemically. |
| 2. | The smallest unit of an element is an atom. | The smallest unit of a compound is a molecule. |
| 3. | A molecule of an element is made up of same kind of atoms. | A molecule of a compound is made up of atoms of two or more elements. |
| 4. | An element has its own set of properties. | The properties of a compound are completely different from the properties of elements from which it is made up of. |
A mixture of sand and ammonium chloride can be separated by :
- filtration
- distillation
- sublimation
- crystallisation
Answer
sublimation
Reason — The mixture is first subjected to sublimation. Ammonium chloride sublimes, leaving behind sand. The vapours of ammonium chloride solidifies after cooling down. Hence, sand and ammonium chloride are separated.
A solvent for iodine is :
- water
- kerosene oil
- alcohol
- petrol
Answer
alcohol
Reason — A solvent for iodine is alcohol. In Solvent extraction method, Iodine is dissolved in alcohol, to separate the components of a mixture.
This gas is highly soluble in water :
- Ammonia
- Nitrogen
- Oxygen
- Carbon monoxide
Answer
Ammonia
Reason — Ammonia (NH₃) is highly soluble in water.
Which of the following methods is used to separate potassium nitrate and sodium chloride from their mixture ?
- distillation
- crystallisation
- fractional distillation
- fractional crystallisation
Answer
fractional crystallisation
Reason — Fractional crystallization is the process of separating solids based on differences in solubility. In a mixture of salt and potassium nitrate, potassium nitrate crystallizes first when the solution is cooled, leaving sodium chloride behind. This process is repeated to separate the two.
Which method is used to separate the components of crude petroleum ?
- distillation
- crystallisation
- chromatography
- fractional distillation
Answer
fractional distillation
Reason — Fractional distillation is used to separate the components of crude petroleum. Since crude oil consists of different hydrocarbons with varying boiling points, fractional distillation helps separate them into fractions such as gasoline, diesel, kerosene, and other petroleum products.
Assertion (A): Elements and compounds are pure substances.
Reason (R): Elements and compounds are made up of only one kind of atoms and molecules respectively.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Since elements and compounds are made up of only one kind of atoms and molecules respectively, they are pure substances.
Assertion (A): Mixtures are impure substances made up of two or more kinds of elements and compounds mixed together in any proportion.
Reason (R): Homogeneous mixtures have a uniform composition throughout their bulk.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation — Assertion (A) defines what a mixture is—two or more substances (elements/compounds) physically combined in any proportion, hence "impure."
Reason (R) states a property of one type of mixture (homogeneous = uniform composition). It doesn’t explain why mixtures are impure or their "any proportion" nature, so it isn’t the explanation for Assertion (A).
Assertion (A): All compounds contain more than one type of element in their molecules.
Reason (R) : Compounds can be homogeneous or heterogeneous.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Compounds are always homogeneous; their composition is uniform throughout.
Assertion (A): Metalloids are the elements showing some properties of metals and some properties of non-metals.
Reason (R): Metalloids are hard solids.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation — Metalloids are elements showing some properties of metals and some properties of non-metals. They are hard solids. But metalloids being hard solids does not explain why they have mixed (metal-like and non-metal-like) properties.
Assertion (A): Chromatography is the latest technique to separate the components of a mixture when all the components are different in their properties.
Reason (R): Components of ink are separated by chromatography.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation — Assertion (A) is incorrect—chromatography isn't the "latest" technique, and separation depends on differential adsorption/partition, not on all components being different in all properties.
Reason (R) is correct—ink components can indeed be separated by chromatography (e.g., paper chromatography).
Fill in the blanks:
(a) Brass is a solid-solid ............... mixture.
(b) The method used to separate sawdust and sand is ............... .
(c) Kerosene oil and water are ............... liquids.
(d) Atomicity is the number of atoms in a molecule of ............... .
Answer
(a) Homogeneous
(b) Gravity separation method
(c) heterogeneous
(d) an element
Write true or false against the following statements .
(a) The elements present in a compound can be obtained by simple physical methods.
(b) The properties of components are retained in a mixture.
(c) Common salt is separated from sea water by evaporation.
(d) A mixture has a definite chemical formula.
Answer
(a) False
Correct Statement — The elements present in a compound cannot be obtained by simple physical methods.
(b) True
(c) True
(d) False
Correct Statement — A mixture has no definite chemical formula.
Match the following columns.
| Column A | Column B |
|---|---|
| (a) Solid + Liquid mixture | (i) Krypton |
| (b) Filtration | (ii) Pure substance |
| (c) Chromatography | (iii) Salt and water |
| (d) Compounds | (iv) Residue |
| (e) Inert gas | (v) Components of ink |
Answer
| Column A | Column B |
|---|---|
| (a) Solid + Liquid mixture | (iii) Salt and water |
| (b) Filtration | (iv) Residue |
| (c) Chromatography | (v) Components of ink |
| (d) Compounds | (ii) Pure substance |
| (e) Inert gas | (i) Krypton |
Classify the following substances into compounds and mixtures:
Carbon dioxide, air, water, milk, common salt, blood, fruit juice, sugar, iron sulphide, brass.
Answer
Compounds — Carbon dioxide, Iron sulphide, Water, Common Salt, sugar.
Mixtures — Air, Milk, Fruit Juice, blood, brass.
Give one example for each of the following types of mixtures.
(a) solid-solid homogeneous mixture
(b) solid-liquid heterogeneous mixture
(c) miscible liquids
(d) liquid-gas homogeneous mixture
Answer
(a) Brass is an example of solid-solid homogeneous mixture.
(b) Sand and water is an example of solid-liquid heterogeneous mixture.
(c) Mixture of Acetone and Water is a miscible liquid.
(d) Ammonia and water is a liquid-gas homogeneous mixture.
Define :
(a) A mixture
(b) Crystallisation
(c) Distillation
Answer
(a) Mixture — Mixtures can be defined as a kind of matter which is formed by mixing two or more pure substances (elements and compounds) in any proportion, such that they do not undergo any chemical change and retain their individual properties.
(b) Crystallisation — Crystallisation is the process of forming solid crystals from a hot super-saturated solution by cooling. It occurs when a substance dissolves in a solvent, and as the solvent evaporates or cools, the solute forms solid crystals. This process is often used to purify substances by separating them from impurities. An example is the crystallization of salt from seawater.
(c) Distillation — Distillation is the process of converting a liquid into vapour by heating and the subsequent condensation of the vapour back into liquid.
Differentiate between a residue and a sediment.
Answer
Difference between a residue and a sediment:
| Residue | Sediment |
|---|---|
| A residue refers to the solid material left behind after a physical or chemical process, such as filtration or evaporation. It remains on the filter paper or in the container after the liquid has been separated. | A sediment refers to solid particles that settle at the bottom of a liquid due to gravity. This can occur when a suspension is allowed to stand, and the heavier particles sink. |
Differentiate between a filtrate and a distillate.
Answer
Difference between a filtrate and a distillate:
| Filtrate | Distillate |
|---|---|
| Filtrate is the liquid that passes through the filter during a filtration process. It contains the dissolved substances or small particles that are not large enough to be trapped by the filter paper. | Distillate is the liquid collected after a distillation process. It is the condensed vapour of a liquid that has been separated based on its boiling point. |
What is the advantage of distillation in comparison to evaporation ?
Answer
The advantages of distillation in comparison to evaporation are:
- Separation of Components: Distillation separates liquids based on boiling points, while evaporation only removes the solvent, leaving the solute behind.
- Collection of Purified Liquid: In distillation, the vapour is condensed back into liquid form, allowing the collection of purified components. Evaporation doesn’t collect the evaporated substance.
- Faster & Controlled Process: Distillation is faster and more controlled, allowing precise separation. Evaporation is slower and less controlled.
- Separation of Volatile Components: Distillation can separate volatile substances, like in fractional distillation of crude oil. Evaporation cannot effectively separate components.
Suggest a suitable technique to separate the constituents of the following mixtures. Also give the reason for selecting the particular method.
(a) Chalk powder from water
(b) Iron from sulphur
(c) Water and alcohol
(d) Sodium chloride and potassium nitrate
(e) Calcium carbonate and sodium chloride
Answer
(a) Chalk powder and water can be separated by filtration method. Filtration method is used for separating insoluble solids from liquids. On filtration, chalk powder gets deposited on filter paper whereas water passes through the filter paper and gets collected in the beaker.
(b) Iron from sulphur can be separated by magnetic separation method. This method is used when one of the component is magnetic in nature. Iron gets attracted towards magnet and can easily be separated from sulphur.
(c) Water and alcohol can be separated by fractional distillation method. Fractional distillation method is used to separate miscible mixtures in which the liquids have different boiling points. Boiling point of alcohol is 78°C and water is 100°C. Hence, this method is the most suitable for separating them. 95.5% pure alcohol is obtained by this method.
(d) Sodium chloride and potassium nitrate can be separated by fractional crystallization. This method is used when the solubility of solid components of a mixture is different in the same solvent. Both salts are soluble in water but solubility of potassium nitrate is more than that of sodium chloride in water.
(e) Solvent extraction method is used to separate calcium carbonate and sodium chloride mixture. This method is used when one component is soluble in a particular liquid while the other component is insoluble. Sodium chloride is soluble in water but calcium carbonate is insoluble which settles down.
List four characteristics of mixtures.
Answer
Four characteristics of mixtures are:
- A mixture consists of two or more pure substances that exist together without any chemical combination between them.
- A mixture may be homogeneous or heterogeneous.
- The components of mixtures vary in their proportions.
- In mixtures, the components are loosely held together and therefore they retain their individual properties.
State four differences between compounds and mixtures.
Answer
| Compounds | Mixtures |
|---|---|
| A compound is a pure substance. | A mixture is an impure substance. |
| Compounds are always homogeneous. | Mixtures may be homogeneous or heterogeneous. |
| Formation of a compound involves a change in energy. | Formation of a mixture does not involve any change in energy. |
| Components of compounds can be separated only by complex chemical processes. | Components of mixtures can be separated by simple physical methods of separation. |
Why is it necessary to separate the components of a mixture ? Explain.
Answer
It is necessary to separate the components of a mixture for the following reasons:
To Obtain Useful Substances: Some mixtures contain valuable substances that can be separated and used for various purposes. For example, when crude petroleum is separated, we obtain useful substances like LPG, CNG, petrol, diesel, and kerosene oil, which are primarily used as fuels.
To Remove Unwanted and Harmful Substances: Mixtures may contain impurities or harmful substances that can be harmful to health or degrade the quality of the product. For example, small stones and husk need to be removed from cereals like rice, wheat, and pulses before cooking because they are harmful.
To Obtain Pure Substances for Preparing Other Useful Substances: Sometimes, it is important to separate unwanted components to obtain a pure substance for specific purposes. For instance, drinking water contains air and mineral salts, which need to be removed to obtain pure water for laboratory use, medicine preparation, or for use in car batteries.
Hence, separating the components of a mixture ensures we obtain useful, pure substances and remove harmful or unnecessary impurities.
How sand, salt and ammonium chloride will be separated from their mixture ? Explain.
Answer
Below procedure can be followed for separating a mixture of sand, salt and ammonium chloride:
- The mixture is first subjected to sublimation. Ammonium chloride sublimes, leaving behind sand and salt. The vapours of ammonium chloride are collected and on cooling down, they solidify.
- Solvent extraction method is used to separate the mixture of sand and salt. Water is added to the mixture. Common salt dissolves while sand settles down. The salt solution is carefully decanted to separate it from sand.
- The solution is then evaporated to obtain salt from water.
(a) What is chromatography ? For which type of mixture is it used ?
(b) What are the advantages of chromatography ?
(c) Give two applications of chromatography.
Answer
(a) The process of separating different dissolved constituents of a mixture by their adsorption on an appropriate material is called chromatography. It is used to separate the components of a mixture when all the components are very similar in their properties. It is used to separate liquid-liquid mixtures.
(b) The advantages of chromatography are:
- A very small quantity of the substance can be separated.
- Components with very similar physical and chemical properties can be separated.
- It identifies the different constituents of a mixture.
- It also helps in quantitative estimation of the components of a mixture.
(c) The two applications of chromatography are:
- It is used to purify many industrial products.
- It is used to separate pigments from natural colors.
Draw a well-labelled diagram to separate kerosene oil from water.
Answer
The mixture of kerosene oil and water is placed in a separating funnel, where they form two layers. Water, being heavier, forms the lower layer, and kerosene oil, being lighter, forms the upper layer. The stopper is opened to drain the water (the heavier liquid) into a vessel, and the process is stopped when the lower layer is completely removed, separating the two liquids.
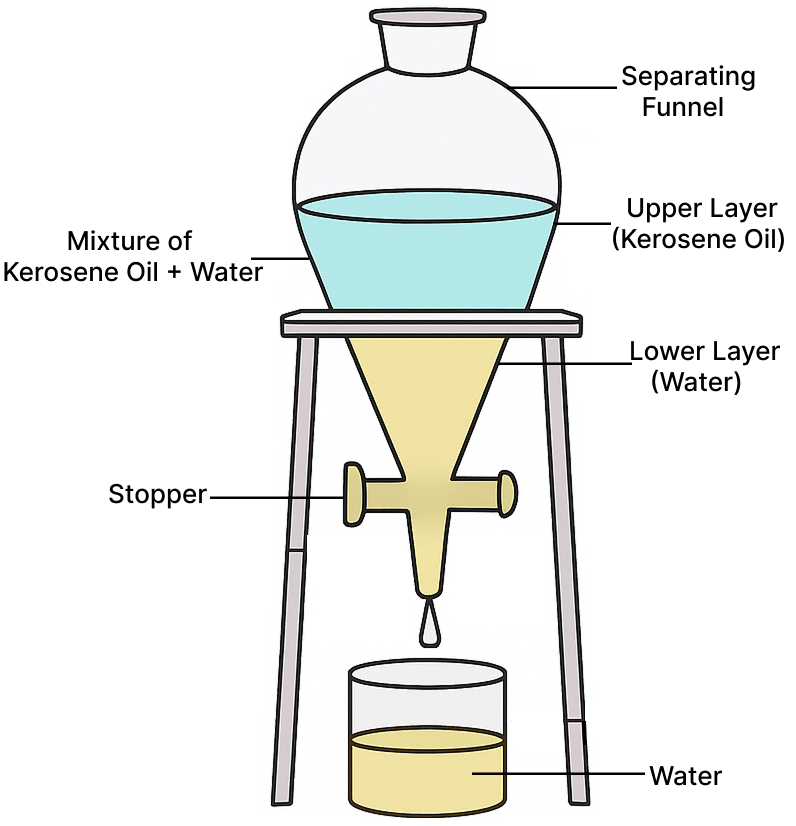
State the principles involved in the following methods used to separate the components of mixtures.
(a) Solvent extraction
(b) Gravity separation
(c) Fractional crystallisation
(d) Fractional distillation
Also give one example of a mixture separated by employing the above methods.
Answer
(a) Solvent extraction — Solvent extraction is based on the difference in solubility of components in a solvent. A solvent is chosen in which one or more components of the mixture dissolve, while others do not. The soluble component is then separated from the insoluble ones.
Example: A mixture of sodium chloride and calcium carbonate can be separated by this method.
(b) Gravity separation — Gravity separation works on the principle of differences in the densities of components. Heavier particles settle at the bottom due to gravity, while lighter particles remain on top or are separated by other means.
Example: A mixture of sand and sawdust can be separated by this method.
(c) Fractional crystallisation — Fractional crystallisation relies on the difference in solubility of components in the same solvent. When a mixture of solids is dissolved in a solvent and the solution is heated, the components may have different solubilities. As the solution cools, the less soluble component crystallizes first, while the more soluble component remains dissolved. Repeating this process allows for the separation of the components.
Example: A mixture of common salt and potassium nitrate can be separated by this method.
(d) Fractional distillation — Fractional distillation is used to separate liquid-liquid homogeneous mixtures (miscible liquids) that have different boiling points. When the mixture is heated, the liquid with the lower boiling point vapourizes first, and its vapour is condensed and collected in a separate receiver. The process is repeated, and the second liquid vapourizes once the temperature rises to its boiling point.
Example: Water and alcohol are separated by this method.