Hydrogen chloride molecule is:
- Polar covalent
- Non polar
- Ionic
- Co-ordinate
Answer
Polar covalent
Reason — Hydrogen chloride (HCl) is a polar covalent molecule because it is formed by sharing of electrons between hydrogen and chlorine atoms. However, chlorine is more electronegative than hydrogen, so it pulls the shared electrons closer to itself. This creates a partial negative charge on chlorine and a partial positive charge on hydrogen, making the molecule polar.
Hydrogen chloride gas being highly soluble in water is dried by
- Anhydrous calcium chloride
- Phosphorous pentoxide
- Quicklime
- Conc. sulphuric acid
Answer
Conc. sulphuric acid.
Reason — Drying agent used for drying should only remove the moisture and not react with it, hence, conc. sulphuric acid is used as the drying agent.
The aim of the Fountain experiment is to prove that:
- HCl turns blue litmus red
- HCl is denser than air
- HCl is highly soluble in water
- HCl fumes in moist air
Answer
HCl is highly soluble in water
Reason — Fountain experiment is performed to show the great solubility of HCl gas in water.
HCl dissolves in toluene (C6H5CH3) and the solution:
P — contains hydronium ions.
Q — can produce carbon dioxide on reacting with sodium carbonate.
R — does not show any acidic property.
Which of the following holds true?
- Only P
- Only Q
- Only R
- Both P and Q
Answer
Only R
Reason — Toluene is an organic solvent and non-polar in nature. When HCl dissolves in toluene, it does not ionize to form hydronium [H3O+] ions. Since there are no free hydrogen ions, the solution does not show acidic properties and also cannot react with sodium carbonate to release carbon dioxide gas. Therefore, only R is correct — the solution does not show any acidic property. b
Assertion (A): HCl is produced by the reaction of hydrogen and chlorine in diffused sunlight.
Reason (R): This reaction is explosive in sunlight.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— HCl is produced, when moist hydrogen gas combines with chlorine in presence of diffused sunlight. Hence, assertion (A) is true.
The formation HCl is a explosive reaction in direct sunlight but it is negligible in dark. So, it is carried out in diffused sunlight. Hence, reason (R) is true.
Therefore, Reason (R) thus provides a valid explanation for why this reaction is occurring in diffused sunlight. Hence, Reason (R) is the correct explanation of assertion
Assertion (A): The Fountain experiment is used to demonstrate the high solubility of HCl gas.
Reason (R): Red litmus solution turns blue in Fountain experiment.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation— The principle of fountain experiment is to show the great solubility of the HCl gas. Hence the assertion (A) is true.
The blue litmus solution turns red due to the acidic nature of hydrogen chloride gas in the Fountain experiment. Hence reason (R) is false.
Assertion (A): HCl gas is collected by downward delivery.
Reason (R): HCl gas is heavier than air.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Hydrogen chloride gas is collected by the downward delivery (upward displacement of air). Hence, the assertion (A) is true.
HCl is collected by downward delivery because it is 1.28 times heavier than air. Hence, reason (R) is true and it is the correct explanation of assertion (A).
Assertion (A): HCl gas fumes in moist air.
Reason (R): HCl gas is highly soluble.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Hydrogen chloride (HCl) gas fumes in moist air because it is highly soluble in water. In the presence of moisture, HCl gas dissolves in water vapour in the air to form tiny droplets of hydrochloric acid, which appear as white fumes. So, both the assertion and the reason are true, and the reason correctly explains the assertion.
Assertion (A): HCl gas dissolves in water as well as organic compounds like toluene.
Reason (R): HCl is a polar covalent compound.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Hydrogen chloride (HCl) is a polar covalent compound, which means it can dissolve in polar solvents like water due to attraction between opposite charges. Interestingly, it can also physically dissolve in some non-polar organic solvents like toluene, but without ionizing. The reason HCl can dissolve in both is due to its polar covalent nature. Hence, both the Assertion (A) and Reason (R) are true, and Reason (R) correctly explains Assertion (A).
Assertion (A): HCl gas is used in the preparation of chlorine and chlorides.
Reason (R): Lead chloride is not produced from HCl.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation— HCl gas dissociates into hydrogen and chlorine, when heated to 500°C and hydrogen chloride reacts with metals that come before hydrogen in the electrochemical series to form corresponding chlorides of those metals. Hence, assertion (A) is true.
When concentrated Hydrochloric acid is oxidised by strong oxidising agent like lead oxide lead chloride is formed. Hence, reason (R) is false.
Pb3O4 + 8HCl (conc.) ⟶ 3PbCl2 + 4H2O + Cl2 ↑
Assertion (A): HCl has sour taste.
Reason (R): HCl is highly soluble in water.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation— Hydrogen chloride has sour (acidic) taste. Hence, the assertion (A) is true.
HCl is highly soluble in water and it can be proved by performing Fountain experiment. Hence, the reason (R) is true.
However, the high solubility of HCl in water doesn't relate to its taste, Hence, the reason (R) is not the correct explanation of assertion (A).
Fill in the blank from the choices in the bracket :
(a) Quicklime is not used to dry HCl gas because CaO is ............... [alkaline/acidic/neutral]
(b) When sodium chloride is heated with concentrated sulphuric acid below 200°C, one of the product formed is ............... (sodium hydrogen sulphate / sodium sulphate / chlorine).
Answer
(a) Quicklime is not used to dry HCl gas because CaO is alkaline.
(b) When sodium chloride is heated with concentrated sulphuric acid below 200°C, one of the product formed is sodium hydrogen sulphate.
Name :
(a) a black metallic oxide which reacts with hydrochloric acid to give a coloured solution.
(b) two colourless gases, which when mixed produce a white solid
(c) two gases which chemically combine to form a liquid.
(d) a chloride which is soluble in excess of ammonium hydroxide.
(e) the chemical in which gold can be dissolved.
(f) the experiment which demonstrates that hydrogen chloride is soluble in water.
(g) the gas produced when chlorine water is exposed to sunlight.
(h) the acid which on mixing with silver nitrate solution produces a white precipitate which is soluble in excess of ammonium hydroxide.
(i) the gas which produces dense white fumes with ammonia gas.
(j) an element which reacts with hydrogen to form compound which is strongly acidic in water.
Answer
(a) Copper oxide (CuO)
(b) Hydrogen chloride and Ammonia
(c) Hydrogen and Oxygen
(d) Silver chloride
(e) Aqua Regia
(f) Fountain experiment
(g) Oxygen gas
(h) Dilute hydrochloric acid
(i) Hydrogen chloride
(j) Chlorine
Explanation
(a) Copper oxide (CuO) is black, reacts with HCl to form blue-coloured copper(II) chloride solution.
CuO + 2HCl ⟶ CuCl2 + H2O
(b) NH3 (g) + HCl (g) ⟶ NH4Cl (white solid)
(c) 2H2 (g) + O2 (g) ⟶ 2H2O (liquid water)
(d) AgCl dissolves in excess NH4OH due to formation of [Ag(NH3)2]+ complex.
AgCl + 2NH4OH ⟶ [Ag(NH3)2]+Cl- + 2H2O
(e) Aqua regia (1 part of conc. HNO3 and 3 parts of conc. HCl) dissolves gold.
(f) Fountain experiment vividly shows HCl gas dissolving in water, creating a fountain effect due to pressure drop.
(g) When chlorine water is exposed to sunlight, chlorine reacts with water to form hypochlorous acid. This acid then breaks apart to give nascent oxygen (O) and hydrochloric acid (HCl). The nascent oxygen combines to form Oxygen gas:
Cl2 + H2 HClO + HCl
HClO HCl + [O] (nascent oxygen)
[O] + [O] ⟶ O2
(h) AgNO3 + HCl ⟶ AgCl (white ppt.) + HNO3 which dissolves in excess NH4OH.
(i) NH3 [g] + HCl [g] ⟶ NH4Cl [s]
(j) H2 + Cl2 ⟶ 2HCl; HCl is strongly acidic in water.
Identify the gas evolved when :
(i) Potassium sulphite is treated with dilute hydrochloric acid.
(ii) Concentrated hydrochloric acid is made to react with manganese dioxide.
Answer
(i) Sulphur dioxide gas
(ii) Chlorine gas
↑
Solution A reacts with an acid B (which gives greenish yellow gas on reacting with oxidising agents like Pb3O4) to give white precipitate C insoluble in nitric acid but soluble in ammonium hydroxide. Name A, B and C.
Answer
A → Silver nitrate
B → Hydrochloric acid
C → Silver chloride
Explanation — Solution A is silver nitrate which reacts with acid B i.e., Hydrochloric acid to give a white precipitate C of AgCl which is insoluble in nitric acid but soluble in ammonium hydroxide.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
AgNO3 + HNO3 ⟶ no reaction
AgCl + 2NH4OH ⟶ [Ag(NH3)2Cl)] + 2H2O
A solution of hydrogen chloride in water is prepared.
The following substances are added to separate portions of the solution:
| S. No. | Substances added | Gas evolved | Odour |
|---|---|---|---|
| 1 | Calcium carbonate | ||
| 2 | Magnesium ribbon | ||
| 3 | Manganese(IV) oxide with heating | ||
| 4 | Sodium sulphide |
Complete the table by writing the gas evolved in case and it's odour.
Answer
| S. No. | Substances added | Gas evolved | Odour |
|---|---|---|---|
| 1 | Calcium carbonate | CO2 | odourless |
| 2 | Magnesium ribbon | H2 | odourless |
| 3 | Manganese(IV) oxide with heating | Cl2 | Pungent smell |
| 4 | Sodium sulphide | H2S | Rotten egg smell |
Complete and balance the following reactions, state whether dilute or conc. acid is used.
(a) NH4OH + HCl ⟶
(b) NaHSO3 + HCl ⟶
(c) Pb(NO3)2 + HCl ⟶
(d) Pb3O4 + HCl ⟶
Answer
(a) NH4OH + HCl (dil.)⟶ NH4Cl + H2O
(b) NaHSO3 + HCl (dil.) ⟶ NaCl + H2O + SO2
(c) Pb(NO3)2 + 2HCl (dil.) ⟶ PbCl2+ 2HNO3
(d) Pb3O4 + 8HCl (conc.) ⟶ 3PbCl2 + 4H2O + Cl2 ↑
State the composition of aqua regia. State which component is the oxidizing agent in aqua regia.
Answer
Aqua regia : 1 part of conc. HNO3 and 3 parts of conc. HCl
Nitric acid present in aqua regia oxidizes HCl to chlorine.
How will the action of dilute hydrochloric acid enable you to distinguish between the following?
(a) Sodium carbonate and sodium sulphite
(b) Sodium thiosulphate and sodium sulphite.
Answer
(a) When Sodium carbonate is treated with dil. HCl, odourless carbon dioxide gas is produced.
Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2 ↑
However, when sodium sulphite is treated with dil.HCl, sulphur dioxide gas with a suffocating odour (burning smell) is produced.
Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 ↑
(b) When sodium thiosulphate is treated with dil. HCl it produces sulphur dioxide gas and yellow sulphur precipitates.
Na2S2O3 + 2HCl ⟶ 2NaCl + S + H2O + SO2 ↑
However, S is not produced when sodium sulphite is treated with dil.HCl.
Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 ↑
MnO2, PbO2 and red lead react with conc. HCl acid liberates Cl2.
What is the common property being shown by these metal oxides?
Answer
It shows that hydrochloric acid (HCl) is oxidized to chlorine (Cl2) by strong oxidizing agents such as MnO2, PbO2 and red lead.
Write an equation for the reactions of hydrochloric acid on :
(a) silver nitrate solution
(b) magnesium foil
(c) caustic soda solution
(d) zinc carbonate
(e) manganese (IV) oxide
(f) copper oxide
Answer
(a) Silver nitrate solution
AgNO3 + HCl ⟶ AgCl (white ppt.) + HNO3
(b) Magnesium foil
Mg + 2HCl ⟶ MgCl2 + H2
(c) Caustic soda solution
NaOH + HCl ⟶ NaCl + H2O
(d) Zinc carbonate
ZnCO3 + 2HCl [dil.] ⟶ ZnCl2 + H2O + CO2 [g.]
(e) Manganese (IV) oxide
↑
(f) Copper oxide
CuO + 2HCl ⟶ CuCl2 + H2O.
Write the balanced equations for the reaction of dilute hydrochloric acid with each of the following:
(a) Iron
(b) Sodium hydrogen carbonate
(c) Iron (II) sulphide
(d) magnesium sulphite
Answer
The balanced equation are :
(a) Iron
Fe + 2HCl (dil.) ⟶ FeCl2 + H2 ↑
(b) Sodium hydrogen carbonate
NaHCO3 + HCl ⟶ NaCl + H2O + CO2 ↑
(c) Iron (II) sulphide
FeS + 2HCl ⟶ FeCl2 + H2S ↑
(d) Magnesium sulphite
MgSO3 + 2HCl ⟶ MgCl2 + H2O + SO2 ↑
How would you distinguish between dilute HCl and dilute HNO3, by addition of only one solution.
Answer
When silver nitrate soln. is added to dil. HCl, curdy white ppt. of silver chloride is formed whereas, there is no reaction when silver nitrate soln. is added to dil. HNO3.
AgNO3 + HCl ⟶ AgCl (white ppt.) + HNO3
AgNO3 + HNO3 ⟶ no reaction
State one appropriate observation when :
(a) Copper sulphide is treated with dilute hydrochloric acid.
(b) A few drops of dil. HCl are added to AgNO3 solution. followed by addition of NH4OH solution.
(c) Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
(d) A small piece of zinc is added to dilute hydrochloric acid.
(e) Dilute HCl is added to sodium carbonate crystals.
(f) Concentrated HCl is added to manganese dioxide.
Answer
(a) A colourless gas having smell of rotten eggs is given off. The gas evolved is H2S
CuS + 2HCl ⟶ CuCl2 + H2S ↑
(b) Curdy white precipitate of silver chloride [AgCl] is obtained, which is soluble in excess of NH4OH.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
AgCl + 2NH4OH ⟶ [Ag(NH3)2Cl)] + 2H2O
(c) White precipitate of PbCl2 is formed which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
(d) Hydrogen gas is evolved with bubbles and it burns with a pop sound
Zn + 2HCl ⟶ ZnCl2 + H2 ↑
(e) Effervescence of CO2 seen which turns lime water milky.
Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2 ↑
(f) When concentrated hydrochloric acid is added to manganese dioxide (MnO2), a greenish-yellow gas (chlorine gas) is evolved with effervescence.
MnO2 + 4HCl (conc.) MnCl2 + 2H2O + Cl2↑
Write balanced chemical equations for the following :
(a) Sodium thiosulphate is reacted with dilute hydrochloric acid.
(b) Calcium bicarbonate reacts with dilute hydrochloric acid.
(c) Conc. hydrochloric acid and potassium permanganate solution.
(d) Action of dilute hydrochloric acid on sodium sulphide.
(e) Action of hydrochloric acid on sodium bicarbonate.
Answer
(a) Na2S2O3 + 2HCl ⟶ 2NaCl + S + H2O + SO2 ↑
(b) Ca(HCO3)2 + 2HCl ⟶ CaCl2 + 2H2O + 2CO2 ↑
(c) 2KMnO4 + 16HCl 2KCl + 2MnCl2 + 8H2O + 5Cl2↑
(d) Na2S + 2HCl ⟶ 2NaCl + H2S [g.] ↑
(e) NaHCO3 + HCl ⟶ NaCl + H2O + CO2 ↑
Give balanced equations with conditions, if any, for the following conversions
(a) Sodium Chloride ⟶ Hydrogen Chloride
(b) Hydrogen Chloride ⟶ Iron (II) chloride
(c) Hydrogen Chloride ⟶ Ammonium chloride
(d) Hydrogen Chloride ⟶ Lead chloride.
Answer
(a) ↑
(b) Fe + 2HCl (dil.) ⟶ FeCl2 + H2 [g]
(c) NH4OH + HCl (dil.)⟶ NH4Cl + H2O
(d) ↑
Explain why
(a) anhydrous HCl is a poor conductor while aqueous HCl is an excellent conductor.
(b) when the stopper of a bottle full of hydrogen chloride gas is opened there are fumes in the air.
(c) a solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while a solution of the same gas in toluene
(i) has no effect on litmus, and
(ii) does not conduct electricity.
(d) thick white fumes are formed when a glass rod dipped in NH4OH is brought near the mouth of a bottle full of HCl gas.
(e) dry hydrogen chloride gas does not affect a dry strip of blue litmus paper, but it turns red in the presence of a drop of water.
(f) hydrogen chloride gas is not collected over water.
(g) dilute hydrochloric acid cannot be concentrated by boiling beyond 22.2%.
(h) Hydrogen chloride gas cannot be dried over quick lime.
Answer
(a) Anhydrous HCl is a gas and does not contain any ions, hence it is a poor conductor of electricity. On the other hand, aqueous HCl (HCl dissolved in water) forms ions in solution, and thus becomes an excellent conductor of electricity.
HCl + H2O ⟶ H3O+ + Cl-
(b) When hydrogen chloride gas is exposed to air, it gives white fumes, due to the formation of hydrochloric acid on dissolving in atmospheric water vapour.
(c) A solution of hydrogen chloride in water gives ions,
HCl + H2O ⟶ H3O+ + Cl-
These ions are responsible for conducting electricity and turning blue litmus red, due to the presence of hydronium [H3O+] ions which make aqueous solution of HCl acidic in nature.
Toluene is an organic solvent and HCl is a covalent compound. A solution of HCl in toluene contains only molecules and not ions due to which it does not conduct electricity. The absence of hydronium [H3O+] ions make the solution neutral and it does not effect litmus paper.
(d) When a glass rod dipped in ammonium hydroxide (NH4OH) is brought near the mouth of a bottle full of HCl gas, then it leads to the formation of dense white fumes due to the production of ammonium chloride.
NH4OH + HCl ⟶ NH4Cl + H2O
(e) Dry hydrogen chloride gas does not contain any ions. Due to the absence of hydronium [H3O+] ions, it is neutral and does not affect a dry strip of blue litmus paper. On the other hand, in the presence of a drop of water, HCl dissolves in it and dissociates into hydronium [H3O+] ions and chloride ions [Cl-]
HCl + H2O ⟶ H3O+ + Cl-
The presence of hydronium [H3O+] ions makes the aqueous solution acidic and blue litmus turns red.
(f) Hydrogen chloride gas is not collected over water since it is highly soluble in water.
(g) Hydrochloric acid forms a constant boiling mixture at 110°C, 22.2% by weight. On boiling further, the mixture evolves out the vapours of both acid and water in the same proportion as in the liquid.
Hence, dilute HCl cannot be concentrated beyond 22.2% by boiling.
(h) Quicklime [CaO] is alkaline in nature and it reacts with HCl forming the respective chloride. Hence, it can't be used as a drying agent.
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following.
(a) Name the acid used. Why is this particular acid preferred to other acids?
(b) Give the balanced equation for the reaction.
(c) Name the drying agent used in drying hydrogen chloride gas.
(d) Phosphorus pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
(e) Why is direct absorption of HCl gas in water not feasible?
(f) What arrangement is done to dissolve HCl gas in water.
Answer
The labelled diagram for the laboratory preparation of hydrogen chloride gas is shown below:

(a) Conc. H2SO4 is used for the preparation of hydrogen chloride gas in the laboratory.
As conc. H2SO4 is non-volatile and has a high boiling point, therefore, it displaces the volatile hydrogen chloride from the salt sodium chloride. Hence, conc. H2SO4 is used as a reactant in the laboratory preparation of HCl from sodium chloride.
(b)
(c) Conc. H2SO4
Reason — Drying agent used for drying should only remove the moisture and not react with it, hence, conc. sulphuric acid is used as the drying agent.
(d) Phosphorus pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas since they react with it.
2P2O5 + 3HCl ⟶ POCl3 + 3HPO3
CaO + 2HCl ⟶ CaCl2 + H2O
(e) Direct absorption of hydrogen chloride gas in water is not feasible because it causes back suction in the delivery tube.
- Hydrogen chloride gas is extremely soluble in water.
- If a delivery tube through which HCl gas is passed is directly immersed in water, the rate of absorption of HCl gas is high and hence a partial vacuum is created in the tube.
- The pressure outside being higher causes the water to be pushed up into the delivery tube and damages the apparatus. This is called back-suction.
(f) A special funnel arrangement is used to dissolve HCl gas in water as it provides a large surface area for absorption while preventing back-suction. The rim of the funnel is positioned so that it just touches the water in the trough. If back-suction occurs, the water rises up the funnel and the air gap between the rim of the funnel and the water's surface equalizes the pressure inside and outside.
The given set up in the figure is for the preparation of an acid

(a) Name the acid prepared by this method.
(b) Name the reactants used.
(c) Why an empty flask is used?
(d) What is the drying agent used? Why is this drying agent chosen ?
(e) What is the role of inverted funnel in the arrangement?
Answer
(a) Hydrochloric acid (HCl)
(b) The reactants used are sodium chloride and sulphuric acid.
(c) The empty flask acts as an Anti-Suction device. If back suction takes place then the water gets collected in it and will not reach the generating flask.
(d) The drying agent used is Concentrated Sulphuric acid. It is chosen as drying agent because it does not react with HCl.
(e) The role of the inverted funnel in the arrangement is -
- It prevents or minimizes back suction of water.
- It provides a large surface area for absorption of HCl gas.
How will you prove that Hydrochloric acid contains
(i) hydrogen
(ii) chlorine.
Write equations for the reactions.
Answer
(i) Using an active metal below magnesium
Fe + 2HCl ⟶ FeCl2 + H2↑
(ii) Using an oxidising agent not containing lead.
MnO2 + 4HCl ⟶ MnCl2 + 2H2O + Cl2↑
Give three distinct tests [apart from using an indicator] you would carry out with solution of HCl to illustrate the typical properties of an acid.
Answer
(i) Action on metals — Hydrochloric acid reacts with metals above hydrogen in the activity series forming metallic chlorides and evolving hydrogen.
Ca + 2HCl ⟶ CaCl2 + H2 ↑
(ii) Action on oxides — Hydrochloric acid reacts with oxides to form salt and water only.
CuO + 2HCl ⟶ CuCl2 + H2O
(iii) With salts of weaker acids — Hydrochloric acid decomposes salts of weaker acids.
Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2 ↑
Convert:
(a) Two soluble metallic nitrates to insoluble metallic chlorides using dil. HCl.
(b) Insoluble iron(II) oxide to a soluble compound.
(c) Insoluble metal carbonate to a soluble chloride.
(d) Metal sulphide to an offensive smelling gas.
Answer
(a) Pb(NO3)2 + 2HCl ⟶ PbCl2 + 2HNO3
Hg2(NO3)2 + 2HCl ⟶ Hg2Cl2 + 2HNO3
(b) FeO + 2HCl ⟶ FeCl2 + H2O
(c) CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2 ↑
(d) Na2S + 2HCl ⟶ 2NaCl + H2S ↑
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E

Answer
The balanced chemical reactions are:
A : ↑
B : ↑
C : AgNO3 + HCl ⟶ AgCl (white ppt.) + HNO3
D : ↑
E : NaHCO3 + HCl ⟶ NaCl + H2O + CO2 ↑
In the laboratory preparation of hydrochloric acid, hydrogen chloride gas is dissolved in water.
(a) Draw a diagram to show the arrangement used for the absorption of HCl gas in water.
(b) State why such an arrangement is necessary. Give two reasons for the same.
(c) Write balanced chemical equations for the laboratory preparation of HCl gas when the reaction is :
(A) below 200°C
(B) above 200°C
Answer
(a) Below diagrams show the special funnel arrangement used for the absorption of HCl gas in water:


(b) The reasons are :
- Prevents or minimizes back-suction of water.
- Provides a large surface area for the absorption of the HCl gas.
(c) The equations are:
(A) ↑
(B) ↑
Study the figure given below and answer the questions that follow :

(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
Answer
(i) Dry HCl (Hydrogen chloride) gas is the gas Y
(ii) High solubility of HCl in water
(iii) Ammonia (NH3) gas also demonstrates high solubility in water.
Refer to the diagram given below and write balanced equations with conditions, if any, for the following conversions P to S.
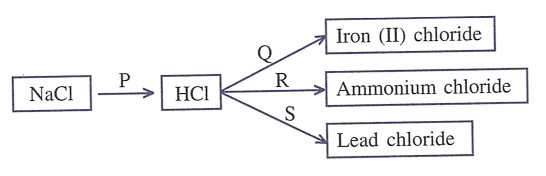
Answer
P — ↑
Q — Fe + 2HCl (dil.) ⟶ FeCl2 + H2 [g]
R — NH4OH + HCl (dil.)⟶ NH4Cl + H2O
S — ↑
State your observations:
(a) HCl gas is passed through lead nitrate solution and the mixture is heated.
(b) Hydrochloric acid is added to silver nitrate solution.
(c) Ammonium hydroxide solution is added to the resultant product of part (b).
Answer
(a) When HCl gas is passed through lead nitrate, white precipitate of lead chloride is formed.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
(b) Hydrochloric acid, when reacted with silver nitrate solution it gives a thick curdy white precipitate of silver chloride. This white precipitate is insoluble in nitric acid.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(c) On addition of ammonium hydroxide, the resultant of part (b) i.e., thick curdy white precipitate of silver chloride dissolves and forms a complex salt called diammine silver (I) chloride.
AgCl + 2NH4OH ⟶ [Ag(NH3)2Cl)] + 2H2O
Distinguish by using HCl.
(a) Lead nitrate solution and silver nitrate solution.
(b) Potassium sulphite and potassium sulphide.
Answer
(a) On reaction with HCl, lead nitrate solution gives white precipitate which can be dissolved in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
Whereas, silver nitrate solution gives thick curdy white precipitate which is insoluble in hot water.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(b) On reaction with HCl, potassium sulphite gives SO2 gas with pungent odour
K2SO3 + 2HCl ⟶ 2KCl + H2O + SO2 ↑
However, potassium sulphide gives H2S gas with rotten eggs odour.
K2S + 2HCl ⟶ 2KCl + H2S ↑
The following questions pertain to the laboratory preparation of hydrogen chloride gas:
(a) Write the equation for it's preparation mentioning the conditions required.
(b) Name the drying agent used in the above preparation and give a reason for the choice.
(c) State a safety precaution taken during the preparation of hydrochloric acid.
Answer
(a) The equation for the laboratory preparation of hydrogen chloride gas :
↑
(b) Concentrated sulphuric acid is used as the drying agent used in the above preparation.
Reason — Drying agent used for drying should only remove the moisture and not react with it, hence, conc. sulphuric acid is used as the drying agent. Other drying agents like phosphorous pentoxide (P2O5) and quick lime (CaO) cannot be used since they react with hydrogen chloride gas.
(c) Temperature is maintained at nearly 200°C as at higher temperatures above 200°C the glass apparatus may crack, fuel is wasted, sodium sulphate formed, forms a hard crust which sticks to the glass and is difficult to remove.
Identify the gas evolved and give the chemical test in each of the following cases.
Dilute hydrochloric acid reacts with:
(i) Iron [II] sulphide.
(ii) Sodium sulphite.
Answer
(i) H2S gas is evolved when dilute hydrochloric acid reacts with Iron [II] sulphide.
FeS + 2HCl ⟶ FeCl2 + H2S ↑
(ii) SO2 is evolved when dilute hydrochloric acid reacts with sodium sulphite.
Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 [g] ↑