Chemistry
A given white crystalline salt was tested as follows :
(a) It dissolved in water and the resulting solution of the salt turned blue litmus red.
(b) Addition of barium chloride solution into this solution gave a white precipitate.
(c) A flame test on the salt gave a persistent golden-yellow colourisation.
What conclusion can be drawn for each observation?
Practical Chemistry
13 Likes
Answer
(a) As the salt solution turned blue litmus red hence the salt may be an acid.
(b) As white ppt. is obtained on addition of barium chloride so the salt may contain SO42-, SO32-, CO32-.
(c) Persistent golden yellow colourisation which suggests presence of Na+ ion.
Answered By
7 Likes
Related Questions
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
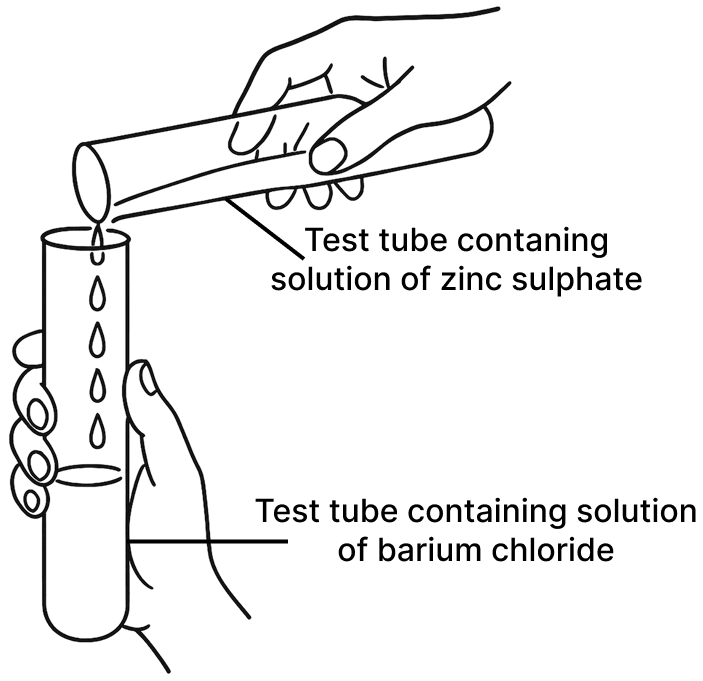
(b) Experiment 2
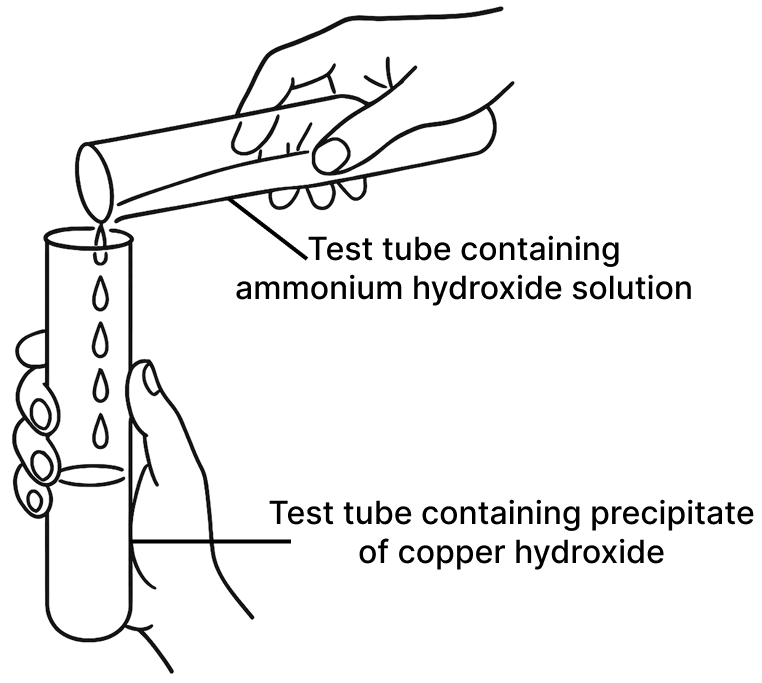
Name the anion present in each of the following compounds.
(a) Compound A when warmed with concentrated sulphuric acid gives a gas which fumes in moist air and which gives dense white fumes with ammonia.
(b) When barium chloride solution is added to a solution of compound B, a white precipitate insoluble in dilute hydrochloric acid is formed.
(c) The action of heat on the insoluble compound C produces a gas which turns lime water turbid.
(d) Compound D when warmed with dilute sulphuric acid gives a gas which turns acidified dichromate solution green.
(a) Sodium hydroxide solution is added to solution A. A white precipitate is formed which is insoluble in excess sodium hydroxide solution. Name the metal ion present in solution A.
(b) When ammonium hydroxide is added to solution B, a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide giving an inky blue solution. Name the cation present in solution B.
(c) When an ammonium salt is warmed with sodium hydroxide solution, ammonia gas is evolved. State three ways in which you could identify this gas.
The questions (i) to (v) refer to the following salt solutions listed A to F.
A. Copper nitrate
B. Iron (II) sulphate
C. Iron (III) chloride
D. Lead nitrate
E. Magnesium sulphate
F. Zinc chloride
(i) Which two solutions will give a white precipitate when treated with dilute hydrochloric acid followed by barium chloride solution?
(ii) Which two solutions will give a white precipitate when treated with dilute nitric acid followed by silver nitrate solution?
(iii) Which solution will give a white precipitate, when either dilute hydrochloric acid or dilute sulphuric acid is added to it?
(iv) Which solution becomes a deep/inky blue colour when excess of ammonium hydroxide is added to it?
(v) Which solution gives a white precipitate with excess ammonium hydroxide solution?