Chemistry
(a) What to you understand by the term acid?
(b) Name the positive ion formed when an acid is dissolved in water.
(c) Draw the structure of this ion.
Acids Bases Salts
312 Likes
Answer
(a) Acids are defined as compounds which contain one or more hydrogen atoms, and when dissolved in water, they produce hydronium ions (H3O+) as the only positively charged ions.
(b) Hydronium ion (H3O+)
(c) The structure of hydronium ion is shown below:
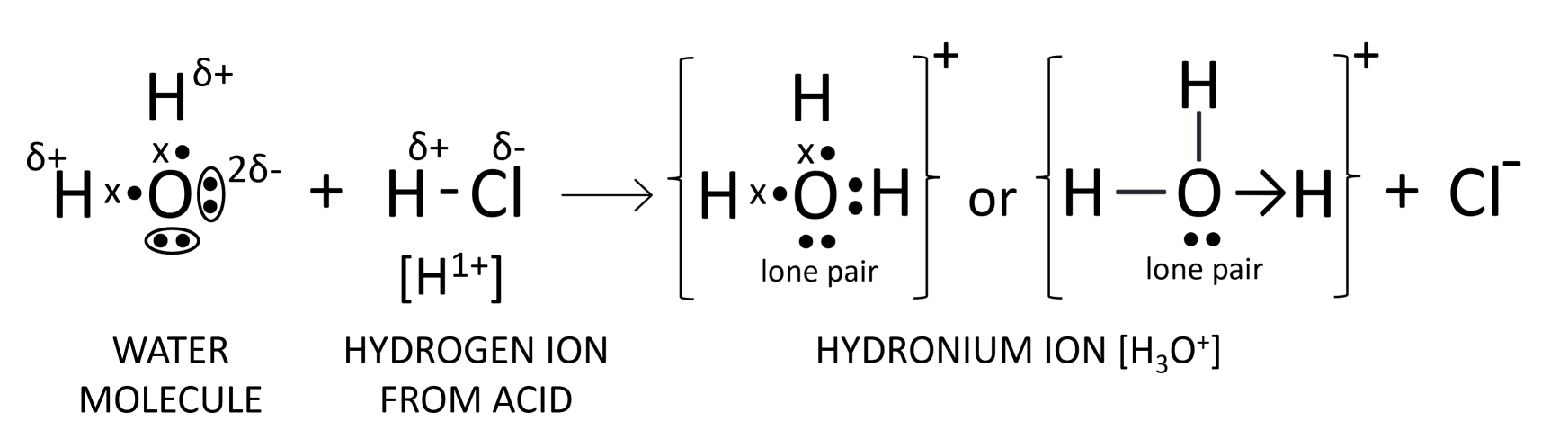
Answered By
214 Likes
Related Questions
Write the ionisation of sulphuric acid showing the formation of hydronium ion.
Water is never added to acid in order to dilute it. Why?
Define the term 'basicity' of an acid. Give the basicity of: nitric acid, sulphuric acid and phosphoric acid.
Give two examples of each of the following :
(a) oxy-acid
(b) hydracid
(c) tribasic acid
(d) dibasic acid