Physics
Calculate the amount of heat absorbed by 200 g of paraffin wax to melt completely at its melting point.
[Specific latent heat of fusion of paraffin wax = 146 Jg⁻¹]
Calorimetry
4 Likes
Answer
Given,
Mass of paraffin (m) = 200 g
Specific latent heat of fusion of paraffin (L) = 146 J g⁻¹
Let, required heat be Q.
Then,
Answered By
3 Likes
Related Questions
According to the NEW colour convention which colour of wire is connected to :
(a) the metal body of the appliance
(b) the switch of the appliance?
(a) Which of the two, alternating current or direct current, produces a varying magnetic field when it flows through a conductor?
(b) State the frequency of the alternating current supply in India
Copper wire is wound around a steel bar FT. Current is allowed to pass through the coil for some time and then the bar is removed.
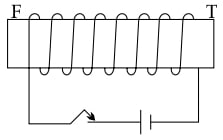
(a) Draw only the magnetised bar FT and mark its poles.
(b) Trace two magnetic lines of force around FT clearly indicating the direction.
A current flows through a metallic conductor for a long period of time. State the change you would expect in its:
(a) Resistance
(b) Resistivity