Chemistry
The below diagram represents the electrolysis of acidulated water. The reaction occurring at the anode is:
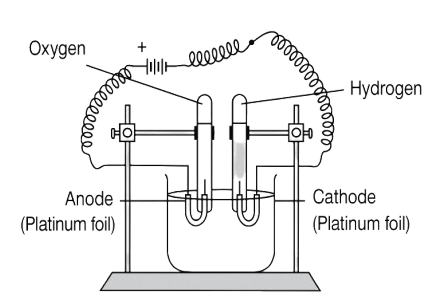
- H2SO4 ⟶ 2H+ + SO42-
- H2O ⟶ H+ + OH-
- H+ + e- ⟶ H, 2[H] + 2[H] ⟶ H2
- OH- - e- → OH, [4OH] ⟶ 2H2O + O2
Electrolysis
2 Likes
Answer
OH- - e- → OH, [4OH] ⟶ 2H2O + O2
Reason — OH- migrates to the anode. OH- being lower in the electro-chemical series is discharged preferentially. OH- loses one electron to the anode and becomes neutral OH.
OH- - e- → OH
The combination of OH forms water with the liberation of oxygen, which is given off at the anode.
[4OH] ⟶ 2H2O + O2
Answered By
1 Like
Related Questions
Which of the following statements about ethane is false?
- It is a saturated hydrocarbon.
- It undergoes a substitution reaction.
- It is a gas at ordinary temperatures.
- It has a triple bond between the carbon atoms.
Thermite mixture is used to weld the broken ends of the iron girders. This mixture consists of ferric oxide and aluminium powder, which, when heated, produces molten iron. In this reaction, the aluminium powder acts as a/an …………… agent.
- oxidising
- reducing
- dehydrating
- corroding
Group number 1A
1IIA
2IIIA
13IVA
14VA
15VIA
16VIIA
17VIIIA
18Li D O J Ne A Mg E Si H K B C F G L With reference to the portion of the periodic table given above, identify the element having the largest atomic size:
- Li
- B
- K
- L
The picture given below shows an apparatus that a teacher used for demonstrating the properties of ionic substances. The teacher heats a sample of lead bromide in a crucible which contains two electrodes which are part of the circuit shown. The bulb does not light up. What is the best explanation for this?
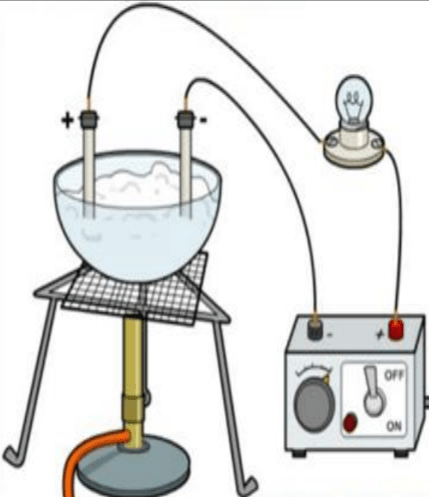
- The circuit is complete.
- Molten lead bromide does not conduct electricity.
- The sample of lead bromide was not heated up to the melting point by the teacher.
- The DC power supply was set up correctly.