Chemistry
Gas M occupies a volume of 1000 c.c and contains X molecules. How many molecules will be present in gas N occupying a volume of 250 c.c?
Mole Concept
4 Likes
Answer
Reason — Let molecules in gas N be Y.
Hence, gas N contains molecules.
Answered By
3 Likes
Related Questions
How many electrons are present in one molecule of CH4?
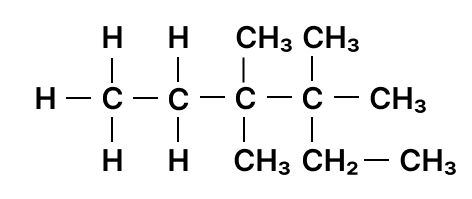
Identify the longest carbon chain and mention the number of carbons present in it.
Element X belongs to period 2 and group 1 of the periodic table. State the formula of the chloride of the element X.
Anurag added dilute H2SO4 to a given sample X and heated the mixture. He observed that a gas was liberated which had a foul smell of rotten eggs and it turned moist lead acetate paper silvery black. Name the gas evolved in the above case.