Physics
On a hot summer day, we often put ice cubes to cool the water. Why?
Calorimetry
1 Like
Answer
Ice is added to warm water on a hot summer day because it absorbs heat from the water to melt. This happens due to the latent heat of fusion, which is 336 J/g, the amount of energy required to change ice from solid to liquid without a rise in temperature. As ice absorbs this heat, the water loses energy, leading to a drop in its temperature and making it cooler.
Answered By
1 Like
Related Questions
In a gold atom (atomic number 79), an electron revolves around the nucleus in a circular orbit. There is a strong electrostatic force between the positively charged nucleus and the negatively charged electron. Though the total positive charge possessed by the nucleus is much higher than the negative charge of the electron, there is no displacement of the electron in the direction of the force.
(a) Name the force responsible for the movement of the electron around the nucleus in its own orbit.
(b) In the absence of such force, what would happen to the movement of the electron?
A straight wire is passed vertically through cardboard sprinkled with iron filings.
(a) When current is passed through the wire in the upward direction, it is seen that the iron fillings are arranging themselves in a definite pattern. Why?
(b) What would happen to this arrangement if more current were passed through the wire?
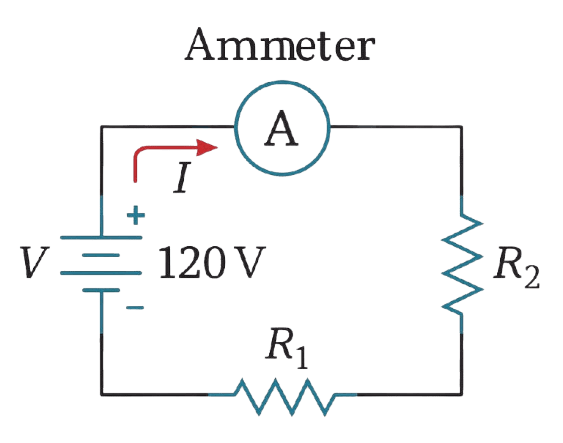
In the given circuit diagram, Minakshi replaced the ammeter by a voltmeter by mistake. Will this circuit work?
A metre rod is half made of copper and half made of iron. If the mass of the copper part is 900 g and the mass of iron is 800 g, then calculate the position at which the rod can remain in equilibrium.