Chemistry
Identify the following:
(a) The compound formed by carbon and hydrogen only.
(b) A substance that do not conduct electricity in molten or aqueous state.
(c) The energy released when an atom in the gaseous state accepts an electron to form an anion.
(d) The name of the process by which aluminium is obtained from alumina.
(e) The bond formed by mutual sharing of a shared pair of electrons.
Chemical Bonding
3 Likes
Answer
(a) Hydrocarbon
(b) Non-electrolyte (Covalent compound)
(c) Electron affinity
(d) Hall-Héroult process (electrolytic reduction)
(e) Covalent bond
Answered By
2 Likes
Related Questions
Match Column A with Column B.
Column A Column B (a) Acid Salt (i) Black in colour (b) Manganese dioxide (ii) Brown ppt. (c) Lead hydroxide (iii) Hydrogen chloride (d) Ferric hydroxide (iv) Calcium hydrogen carbonate (e) Polar compound (v) Soluble in excess sodium hydroxide Complete the following by choosing the correct answers from the bracket:
(a) HCl in the liquefied form is …………… [neutral / acidic]
(b) Organic compounds are generally soluble in ……………[Water / Organic solvents]
(c) An inert electrode used in electrolysis of copper sulphate solution is …………… [Copper/platinum]
(d) Hydrocarbons having triple bond is …………… [alkenes/alkynes]
(e) An acidic gas gives dense white fumes of …………… [NH4OH/NH4C1] with ammonia.
(a) Draw the structure formula for the following :
- 2-pentanal
- 2-methyl butanol
- 1-butyne
(b) Name the following organic compound in IUPAC system :
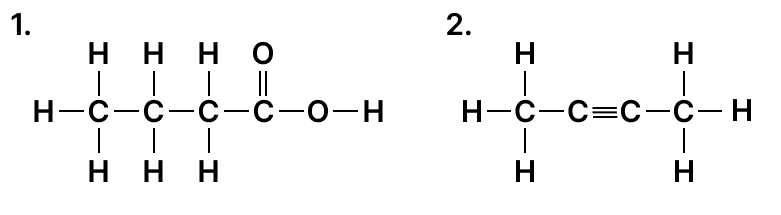
Arrange the following as per the instruction given in the brackets:
(a) Na, K, Cl, Si, S (increasing order of ionisation potential)
(b) Be, Li, F, C, B, N, O (increasing order of non-metallic character)