Physics
A metal piece of mass 5 g has thermal capacity 2.5 J K⁻¹. If the mass of the metal is tripled, then its specific heat capacity will be :
- 7.5 J K⁻¹
- 2.5 J K⁻¹
- 1.5 J g⁻¹ K⁻¹
- 0.5 J g⁻¹ K⁻¹
Calorimetry
7 Likes
Answer
0.5 J g⁻¹ K⁻¹
Reason — As,
Answered By
5 Likes
Related Questions
A ray of light is incident normally on a face of an equilateral prism. The ray gets totally reflected at the second refracting surface. The total deviation produced in the path of the ray is:
- 30°
- 60°
- 90°
- 120°
In a closed circuit containing a bulb and a cell, the electromotive force (ε) and the terminal voltage (V) is related as.
(Given I is current and r is internal resistance.)
- V = ε + Ir
- V = ε – Ir
- V = ε ÷ Ir
- V = ε × Ir
Assertion (A) : As the level of water in a tall measuring cylinder kept under running tap rises, the pitch of sound gradually increases.
Reason (R) : Frequency of sound is inversely proportional to the length of the water column.
- Both (A) and (R) are true and (R) is correct explanation of (A).
- Both (A) and (R) are true and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
In the given circuits Y and Z, the resistors, R1 and R2, are connected in :
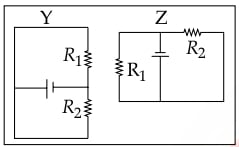
- series in both the circuits
- parallel in both the circuits
- parallel in Y and series in Z
- series in Y and parallel in Z