Chemistry
Assertion (A): Electrovalent compounds conduct electricity even in solid state.
Reason (R): Electrovalent compounds are composed of ions.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Chemical Bonding
2 Likes
Answer
A is false but R is true.
Explanation — Electrovalent compounds cannot disssociate into ions in solid state hence they do not conduct electricity but they are very good conductors of electricity in the molten or in aqueous state. Hence the Assertion (A) is false.
Electrovalent compounds are composed of ions that are held by strong electrostatic force of attraction, which cannot be seperated easily. Hence Reason (R) is true.
Answered By
3 Likes
Related Questions
Draw an electron dot diagram of the structure of — hydronium ion. State the type of bonding present in it.
By drawing an electron dot diagram, show the lone pair effect leading to the formation of — ammonium ion from ammonia gas and hydrogen ion.
Study the extract of the Periodic table given below and answer the questions that follow. Give the alphabet corresponding to the element in question: DO NOT repeat an element.
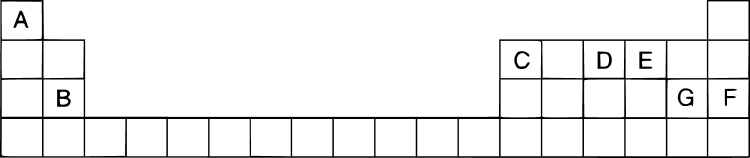
(a) Which element forms an electrovalent compound with G?
(b) The ion of which element will migrate towards the cathode during electrolysis?
(c) Which non metallic element has the valency 2?
(d) Which is the inert gas?
The following table shows the electronic configuration of the elements W, X, Y, Z :
Element W X Y Z Electronic
Configurations2, 8, 1 2, 8, 7 2, 5 1 Answer the following questions based on the table above:
(i) What type of bond is formed between:
- W and X
- Y and Z
(ii) What is the formula of the compound formed between :
- X and Z
- W and X