Chemistry
Assertion (A): Hall Heroult’s process is used to get pure aluminium from its oxide.
Reason (R): Aluminium generally is not found in aluminium oxide form.
- Both A and R are correct.
- A is correct, but R is not a true explanation of A.
- A is correct, and R is a true explanation of B.
- Both A and R are incorrect.
Metallurgy
3 Likes
Answer
Both A and R are correct.
Reason — The Hall-Heroult process is used to extract pure aluminium metal by the electrolytic reduction of aluminium oxide (Al2O3), which is obtained from bauxite by bayer's process. Hence, the assertion (A) is true.
Aluminium is generally not found in aluminium oxide form, aluminum is highly reactive and is found in its main ore bauxite (Al2O3.2H2O). Hence, the reason (R) is correct.
Answered By
2 Likes
Related Questions
Study the below diagram and choose the correct option related to the content given below:
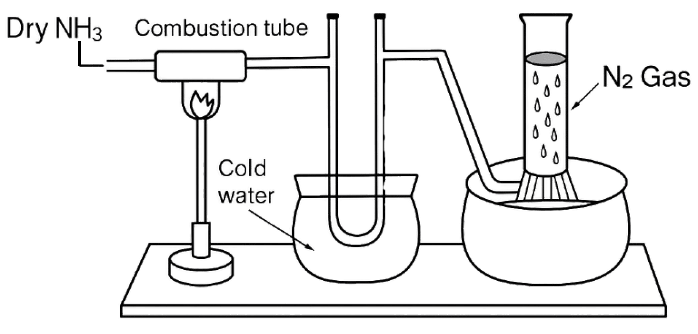
Compound X reacts with ammonia in the combustion tube, which leaves a residue Y. Identify X and Y, as well as the property Z of ammonia demonstrated in this particular reaction.
- X= CuO, Y=black, Z = reducing property.
- X=PbO, Y = yellow, Z=oxidising property.
- X=CuO, Y =yellow, Z =oxidising property.
- X=PbO, Y=black, Z=reducing property.
Assertion (A): Few drops of dilute acid is added to a solution of zinc sulphide, a colourless gas is formed with a rotten egg odour.
Reason (R): Gas formed does not turn moist lead acetate paper silvery black.
- Both A and R are true.
- A and R are true, but R is the correct explanation of A.
- A is true, but R is not the correct explanation of A.
- Both A and R are false.
Assertion (A): Alkenes, alkynes and alkanes are examples of homologous series.
Reason (R): Organic compounds of the homologous series have similar structures but different chemical properties.
- Both A and R are true.
- Both A and R are false.
- A is true but R is not the correct explanation of A.
- A is false but R is true.
Assertion (A): The atomic mass of oxygen is 16 a.m.u; therefore, its gram atomic mass is 16g.
Reason (R): The atomic mass of an element expressed in grams is called gram atomic mass.
- A is true, and R is the correct explanation of A.
- Both A and R are true, but R is not a true explanation of A.
- Both A and R are false.
- R is false, but A is a true explanation.