Chemistry
Assertion (A): The RMM of a gas is 64, then its vapour density is 32.
Reason (R): RMM is twice the vapour density.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Stoichiometry
1 Like
Answer
Both A and R are true and R is the correct explanation of A.
Reason — Vapour density (V.D.) is related to relative molecular mass by:
Substituting the given value:
Thus the assertion is correct. Because the reason states the same relationship—“The RMM is twice the vapour density”—it correctly explains why the vapour density is 32.
Answered By
3 Likes
Related Questions
Nitrogen gas can be obtained by heating
- Ammonium nitrate
- Ammonium nitrite
- Magnesium nitride
- Ammonium chloride
Which of the following figures correctly describes electrorefining ?
- 1.
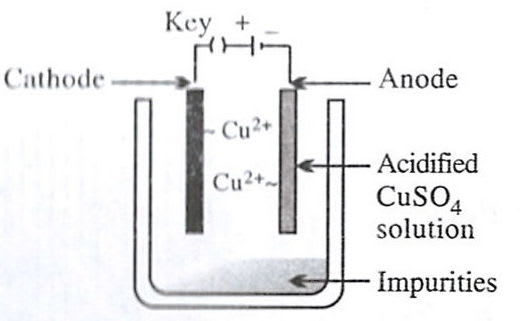
- 2.
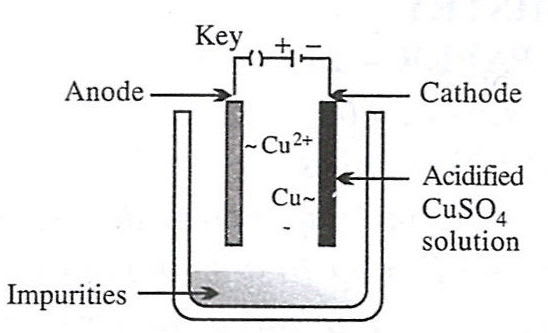
- 3.
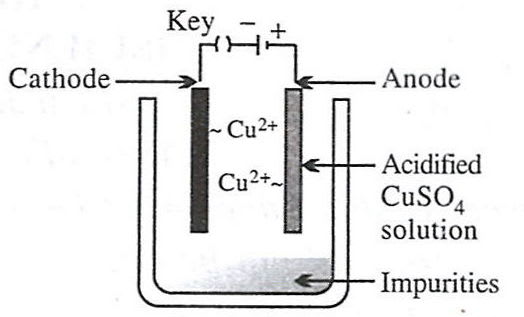
- 4.
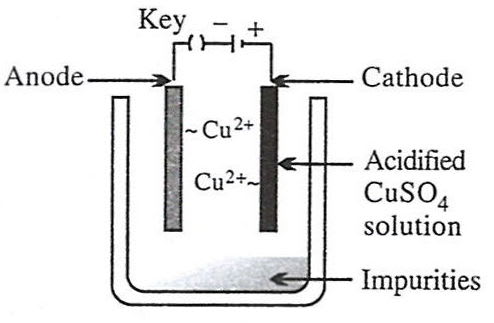
The metals zinc and tin are present in the alloy
- Solder
- Brass
- Bronze
- Duralumin
The organic compound prepared when acetylene reacts with excess of hydrogen:
- Ethane
- Ethene
- Methane
- Propane