Physics
(a) Complete the following radioactive reaction :
(b) Uranium is available in two forms U-235 and U-238. Which of the two isotopes of Uranium is more fissionable?
Radioactivity
6 Likes
Answer
(a)
(b) Comparatively speaking, U-235 is easier to fission than U-238 because only fast neutrons can cause the fission of U-238, whereas slow neutrons can even trigger the fission of U-235.
Answered By
3 Likes
Related Questions
Given are two pulleys.
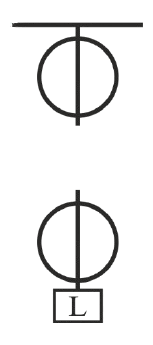
(a) Copy and complete the labelled diagram connecting the two pulleys with a tackle to obtain Velocity Ratio = 2.
(b) If Load = 48 kgf and efficiency is 80% then calculate :
- Mechanical Advantage.
- Effort needed to lift the load
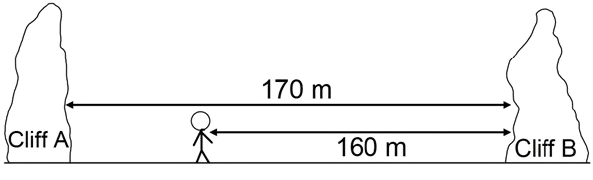
(a) Name the waves used in SONAR.
(b) In the below diagram Lata stands between two cliffs and claps her hands. Determine the time taken by her to hear the first echo. Speed of sound in air = 320 ms-1.
In the given diagram, a vibrating tuning fork is kept near the mouth of a burette filled with water. The length of the air column is adjusted by opening the tap of the burette. At a length of 5 cm of the air column, a loud sound is heard.
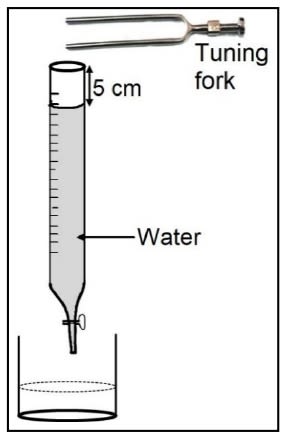
(a) Name the phenomenon illustrated by the above experiment.
(b) Why is a loud sound heard at this particular length?
(c) If the present tuning fork is replaced with a tuning fork of higher frequency, should the length of the air column increase or decrease to produce a loud sound? Give a reason.
The voltage - current readings of a certain material are shown in the table given below :
Voltage (V) Current (I) 10 V 2 A 20 V 3 A 30 V 4 A Study the table.
(a) State whether the conductor used is ohmic or non-ohmic.
(b) Justify your answer.
(c) State Ohm’s law.