Chemistry
Complete the following table and write your observations.
| Hydrogen sulphide | Ammonia | Sulphur dioxide | Hydrogen chloride | |
|---|---|---|---|---|
| Shake the gas with red litmus solution | ||||
| Shake the gas with blue litmus solution | ||||
| Apply a burning splint to the gas |
Practical Chemistry
9 Likes
Answer
| Hydrogen sulphide | Ammonia | Sulphur dioxide | Hydrogen chloride | |
|---|---|---|---|---|
| Shake the gas with red litmus solution | No change | Red litmus becomes blue | No change | No change |
| Shake the gas with blue litmus solution | Blue litmus becomes red | No change | Blue litmus becomes red | Blue litmus becomes red |
| Apply a burning splint to the gas | Burning splint is extinguished | Burning splint is extinguished | Burning splint is extinguished | Burning splint is extinguished |
Answered By
6 Likes
Related Questions
(a) What are soaps and detergents?
(b) Why do they differ in their actions ?
(c) Explain their cleansing actions.
Compare the effect of soap and detergent on hard water.
Copy and complete the following table that refers to the action of heat on some carbonates:
Carbonate Colour of residue on cooling Zinc carbonate Lead carbonate Copper carbonate A series of experiments were performed as shown below to prepare certain gases in the laboratory.
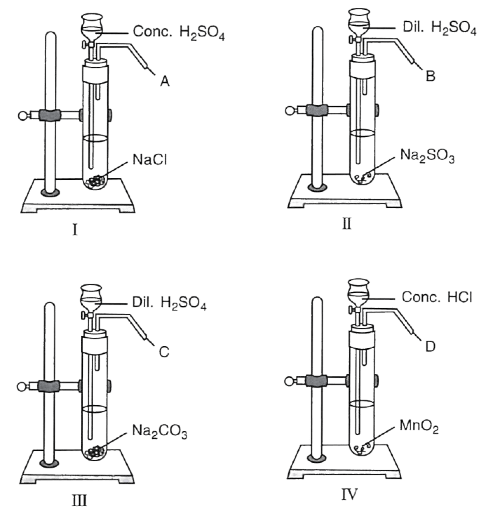
(i) Name the gas A. What do you observe when gas A is passed through AgNO3 solution ?
(ii) Name the gas B. What test will you perform to identify gas B.
(iii) What is the similarity between gas B and gas C ?
(iv) Give a test to distinguish between gases B and C.
(v) In experiment IV, gas D is :
P — HCl
Q — Cl2
R — O2- Only P
- Only Q
- Only R
- Both P and Q
(vi) Write the equations for when gas B and gas C are separately passed in lime water first in little quantities and then in excess.
(vii) When a rod dipped in ammonium hydroxide solution is brought near gas …………… dense white fumes are seen.
P — Gas A
Q — Gas B
R — Gas D- Only P
- Only Q
- Only R
- Both P and R