Chemistry
A series of experiments were performed as shown below to prepare certain gases in the laboratory.
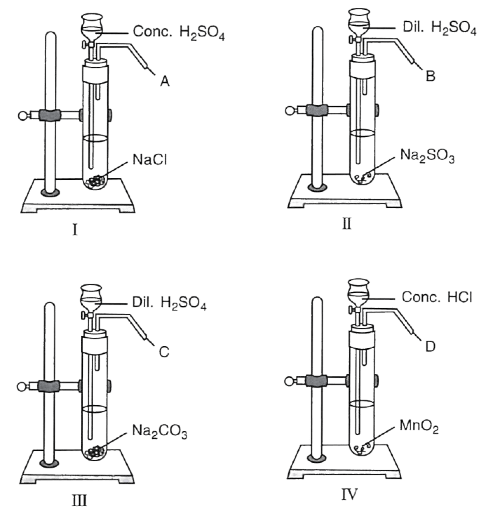
(i) Name the gas A. What do you observe when gas A is passed through AgNO3 solution ?
(ii) Name the gas B. What test will you perform to identify gas B.
(iii) What is the similarity between gas B and gas C ?
(iv) Give a test to distinguish between gases B and C.
(v) In experiment IV, gas D is :
P — HCl
Q — Cl2
R — O2
- Only P
- Only Q
- Only R
- Both P and Q
(vi) Write the equations for when gas B and gas C are separately passed in lime water first in little quantities and then in excess.
(vii) When a rod dipped in ammonium hydroxide solution is brought near gas …………… dense white fumes are seen.
P — Gas A
Q — Gas B
R — Gas D
- Only P
- Only Q
- Only R
- Both P and R
Practical Chemistry
1 Like
Answer
(i) Gas A is HCl, When the HCl is passed through silver nitrate solution, a white precipitate of silver chloride is formed.
AgNO3 (aq) + HCl ⟶ AgCl ↓ + HNO3
(ii) Gas B is SO2 , it decolorises purplish pink acidified potassium permanganate solution.
(iii) Gas B is SO2 and Gas C is CO2, they are colourless, acidic gases which can turn lime water milky (turbid).
(iv) Gas B (SO2) decolorises purplish pink acidified potassium permanganate solution and it can change orange/yellow solution of acidified potassium dichromate green. Gas C (CO2) has no effect on acidified potassium permanganate solution and acidified potassium dichromate solution.
(v) Only Q,
MnO2 + 4HCl ⟶ MnCl2 + H2O + Cl2
(vi)
CO2 in limited Quantity:
CO2 passed in excess:
SO2 in limited Quantity:
SO2 passed in excess:
(vii) Only P
When a glass rod dipped in ammonia solution and brought near the gas A i.e., HCl, dense white fumes of ammonium chloride are formed.
NH3 (aq) + HCl ⟶ NH4Cl
Answered By
3 Likes
Related Questions
(a) What are soaps and detergents?
(b) Why do they differ in their actions ?
(c) Explain their cleansing actions.
Compare the effect of soap and detergent on hard water.
Copy and complete the following table that refers to the action of heat on some carbonates:
Carbonate Colour of residue on cooling Zinc carbonate Lead carbonate Copper carbonate Complete the following table and write your observations.
Hydrogen sulphide Ammonia Sulphur dioxide Hydrogen chloride Shake the gas with red litmus solution Shake the gas with blue litmus solution Apply a burning splint to the gas