Chemistry
When electrolysis of molten lead bromide is carried out, the products formed at the respective electrodes are:
| At the positive electrons | At the negative electrode | |
|---|---|---|
| 1. | Bromine | Lead |
| 2. | Bromine | Hydrogen |
| 3. | Lead | Bromine |
| 4. | Lead | Oxygen |
Electrolysis
2 Likes
Answer
- At the positive electrode — Bromine
At the negative electrode — Hydrogen
Reason — During electrolysis of molten lead bromide,
At the positive electrode — Dark reddish brown fumes of bromine is evolved
Br1- - 1e- ⟶ Br
Br + Br ⟶ Br2
At the negative electrode — Silver gray metal lead is formed.
Pb2+ + 2e- ⟶ Pb
Answered By
1 Like
Related Questions
During the extraction of aluminium by Hall Heroult’s process, the carbon rods are replaced continuously. This is because:
- It minimises heat loss by radiation.
- It enhances the mobility of ions.
- The carbon anode is consumed.
- It lowers the fusion point.
Which of the following observations correctly shows the action of indicator on sodium hydroxide solution?
Indicator methyl orange phenolphthalein 1. P orange to yellow remains colourless 2. Q orange to pink remains colourless 3. R orange to yellow colourless to pink 4. S remains orange remains pink The following are the structural diagrams of certain hydrocarbons:
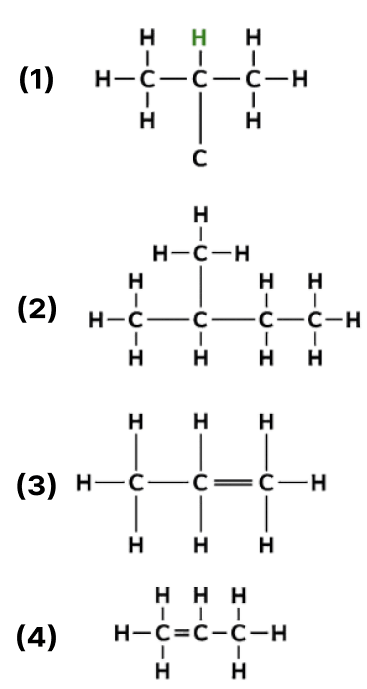
Which two structures are related to each other?
- A and B
- B and C
- C and D
- A and C
The electronic configuration of X is 2,8,6. It gains 'Y’ electrons into its valence shell to attain the nearest noble gas electronic configuration and gets converted to an ion Z. X, Y, and Z, respectively, are:
- Sodium, one, electropositive
- Beryllium, two, electronegative
- Oxygen, six, electronegative
- Sulphur, two, electronegative