Chemistry
Give a reason for each of the following:
(i) Ionic compounds have a high melting point.
(ii) Inert gases do not form ions.
(iii) Ionisation potential increases across a period, from left to right.
(iv) Alkali metals are good reducing agents.
(v) Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
Chemical Bonding
ICSE 2018
13 Likes
Answer
(i) Ionic compounds have ionic bonds so there exists a strong force of attraction between the oppositely charged ions, so a large amount of energy is required to break the strong bonding between the ions. Hence, ionic compounds have a high melting point.
(ii) Inert gases have completely filled octet which makes them extremely stable. They neither lose, nor gain electrons. Hence, they do not form ions.
(iii) The ionisation potential of an element increases across a period because the atomic size decreases due to an increase in nuclear charge and electrons in the outermost shell are more strongly held because of which greater energy is required to remove the electron.
(iv) Alkali metals have one electron in the outermost shell and hence during reactions they readily give the valence electron and get themselves oxidised. Hence, they are good reducing agents.
(v) Dil. H2SO4 is a strong electrolyte and acetic acid is a weak electrolyte. Therefore, dil. H2SO4 allows large amount of electricity to flow through it and is a good conductor of electricity whereas acetic acid allows small amount of electricity to flow through it and is a poor conductor of electricity. Hence, electrical conductivity of dilute hydrochloric acid is greater than that of acetic acid.
Answered By
8 Likes
Related Questions
(i) Give the IUPAC name for each of the following:
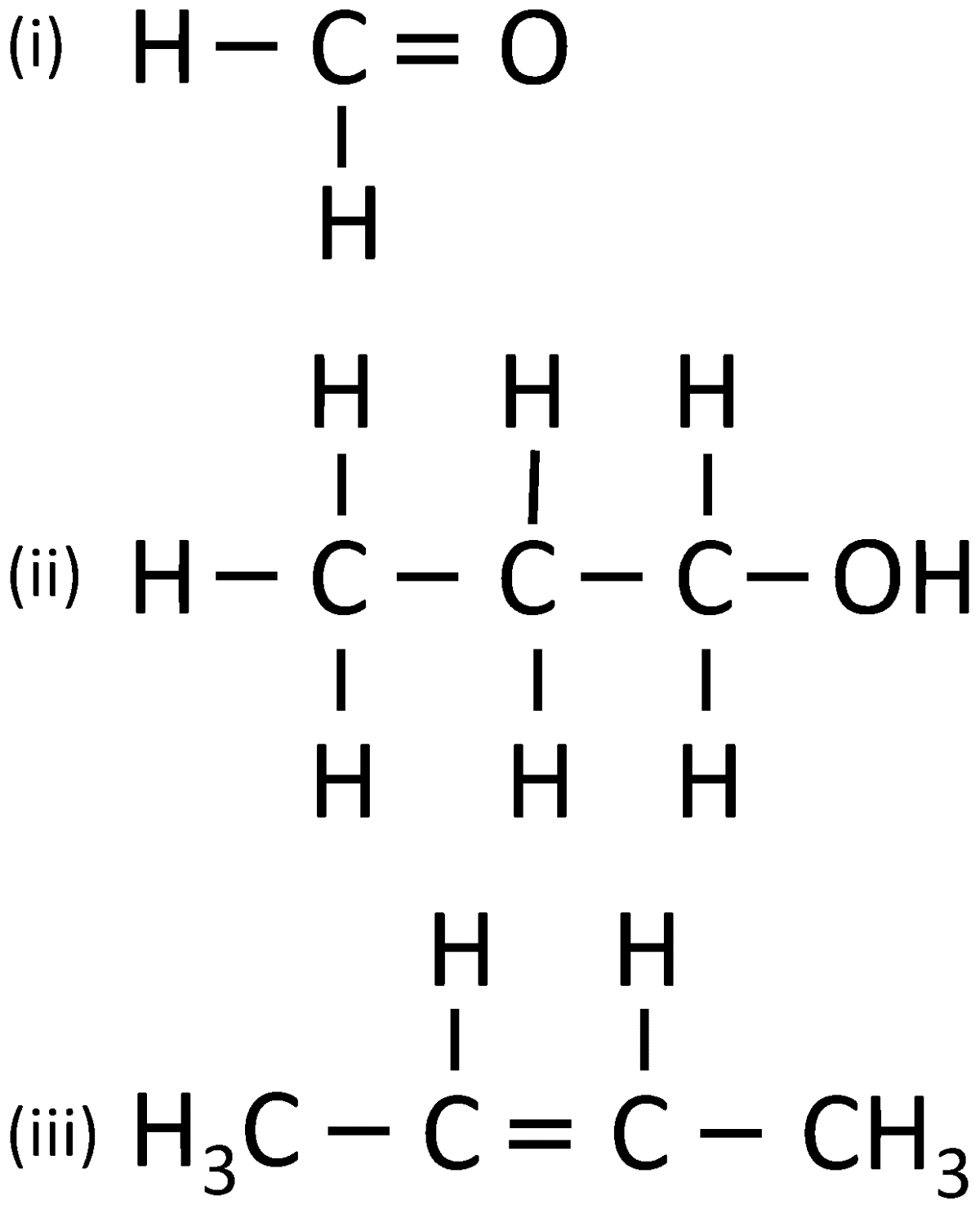
(ii) Write the structural formula of the two isomers of butane.
State one relevant observation for each of the following:
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
(ii) At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
(iii) Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
(iv) Anhydrous calcium chloride is exposed to air for some time.
(v) Barium chloride solution is slowly added to sodium sulphate solution.
Name the gas that is produced in each of the following cases:
(i) Sulphur is oxidized by concentrated nitric acid.
(ii) Action of dilute hydrochloric acid on sodium sulphide.
(iii) Action of cold and dilute nitric acid on copper.
(iv) At the anode during the electrolysis of acidified water.
(v) Reaction of ethanol and sodium.
Fill up the blanks with the correct choice given in brackets.
(i) Ionic or electrovalent compounds do not conduct electricity in their …………… state. (fused / solid)
(ii) Electrolysis of aqueous sodium chloride solution will form …………… at the cathode. (hydrogen gas / sodium metal)
(iii) Dry hydrogen chloride gas can be collected by…………… displacement of air. (downward / upward)
(iv) The most common ore of iron is …………… (calamine / haematite)
(v) The salt prepared by the method of direct combination is …………… (iron (II) chloride / iron (III) chloride)