Chemistry
State one relevant observation for each of the following:
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
(ii) At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
(iii) Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
(iv) Anhydrous calcium chloride is exposed to air for some time.
(v) Barium chloride solution is slowly added to sodium sulphate solution.
Electrolysis
ICSE 2018
14 Likes
Answer
(i) Chalky white precipitate is formed which is soluble in excess of sodium hydroxide and forms a colourless solution.
(ii) At the anode, brown fumes of bromine vapours are observed.
(iii) White precipitate of PbCl2 is formed which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 + 2HNO3
(iv) It absorbs moisture from the atmosphere, dissolve in the same and change into a solution. This property is known as deliquescence
(v) A white ppt. is obtained which is insoluble in dil. HCl or dil. HNO3
Na2SO4 + BaCl2 ⟶ BaSO4 ↓ [white ppt.] + 2NaCl
Answered By
7 Likes
Related Questions
Write a balanced chemical equation for each of the following:
(i) Action of concentrated sulphuric acid on carbon.
(ii) Reaction of sodium hydroxide solution with iron (III) chloride solution.
(iii) Action of heat on aluminum hydroxide.
(iv) Reaction of zinc with potassium hydroxide solution.
(v) Action of dilute hydrochloric acid on magnesium sulphite.
(i) Give the IUPAC name for each of the following:
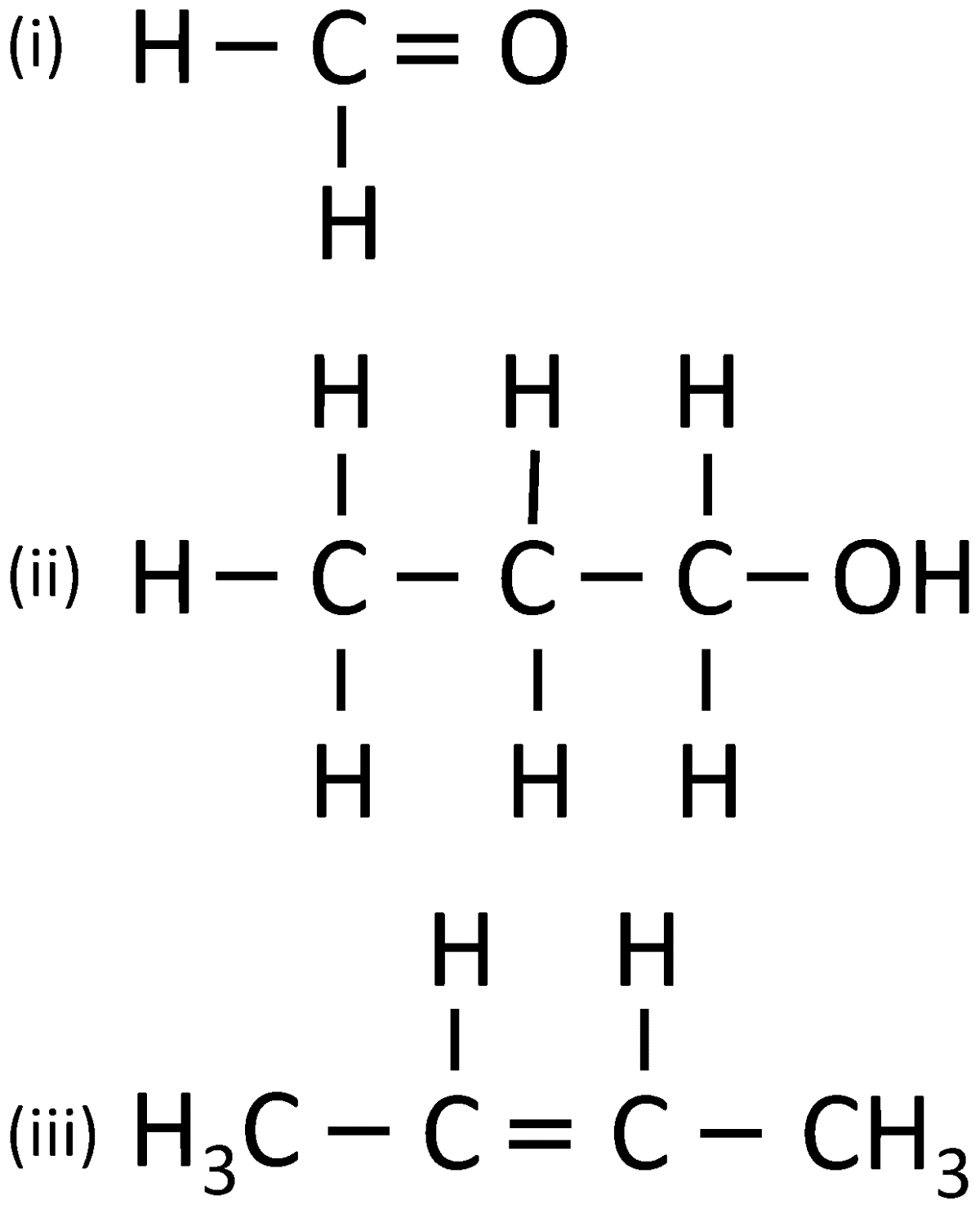
(ii) Write the structural formula of the two isomers of butane.
Give a reason for each of the following:
(i) Ionic compounds have a high melting point.
(ii) Inert gases do not form ions.
(iii) Ionisation potential increases across a period, from left to right.
(iv) Alkali metals are good reducing agents.
(v) Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
Name the gas that is produced in each of the following cases:
(i) Sulphur is oxidized by concentrated nitric acid.
(ii) Action of dilute hydrochloric acid on sodium sulphide.
(iii) Action of cold and dilute nitric acid on copper.
(iv) At the anode during the electrolysis of acidified water.
(v) Reaction of ethanol and sodium.