Chemistry
Write a balanced chemical equation for each of the following:
(i) Action of concentrated sulphuric acid on carbon.
(ii) Reaction of sodium hydroxide solution with iron (III) chloride solution.
(iii) Action of heat on aluminum hydroxide.
(iv) Reaction of zinc with potassium hydroxide solution.
(v) Action of dilute hydrochloric acid on magnesium sulphite.
Acids Bases Salts
ICSE 2018
10 Likes
Answer
(i) C + 2H2SO4 [conc.] ⟶ CO2 + 2H2O + 2SO2
(ii) FeCl3 + 3NaOH ⟶ 3NaCl + Fe(OH)3
(iii) 2Al(OH)3 Al2O3 + 3H2O
(iv) Zn + 2KOH ⟶ K2ZnO2 + H2
(v) MgSO3 + 2HCl ⟶ MgCl2 + H2O + SO2 [g]
Answered By
5 Likes
Related Questions
The catalyst used in the Contact Process is:
- Copper
- Iron
- Vanadium pentoxide
- Manganese dioxide
Give one word or a phrase for the following statements:
(i) The energy released when an electron is added to a neutral gaseous isolated atom to form a negatively charged ion.
(ii) Process of formation of ions from molecules which are not in ionic state.
(iii) The tendency of an element to form chains of identical atoms.
(iv) The property by which certain hydrated salts, when left exposed to atmosphere, lose their water of crystallization and crumble into powder.
(v) The process by which sulphide ore is concentrated.
(i) Give the IUPAC name for each of the following:
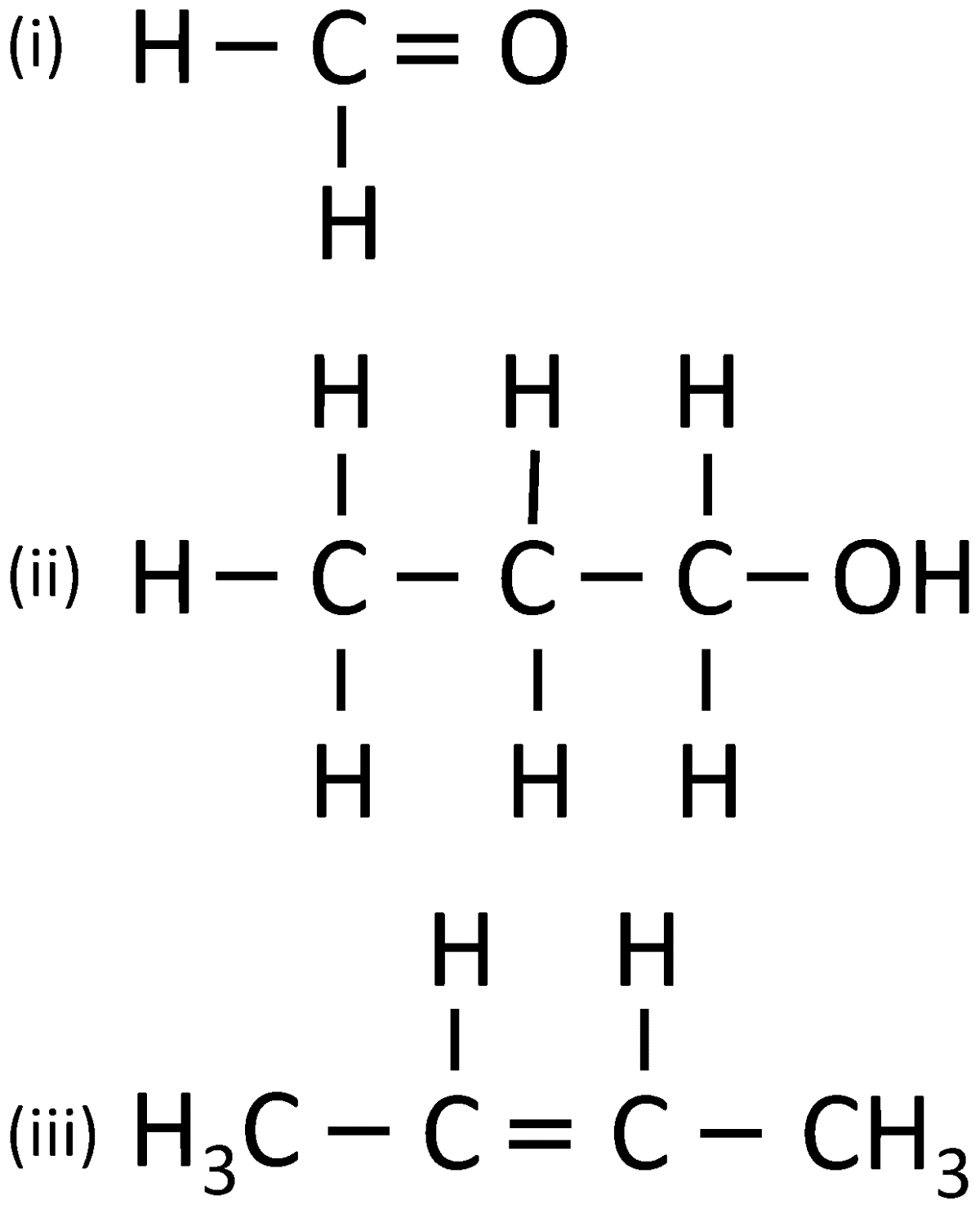
(ii) Write the structural formula of the two isomers of butane.
State one relevant observation for each of the following:
(i) Lead nitrate solution is treated with sodium hydroxide solution drop wise till it is in excess.
(ii) At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
(iii) Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
(iv) Anhydrous calcium chloride is exposed to air for some time.
(v) Barium chloride solution is slowly added to sodium sulphate solution.