Physics
The graph illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for element C in the periodic table. The element is denoted by letters rather than their conventional symbols. When element C, depicted in the graph, undergoes radioactive decay, it releases radioactive rays.
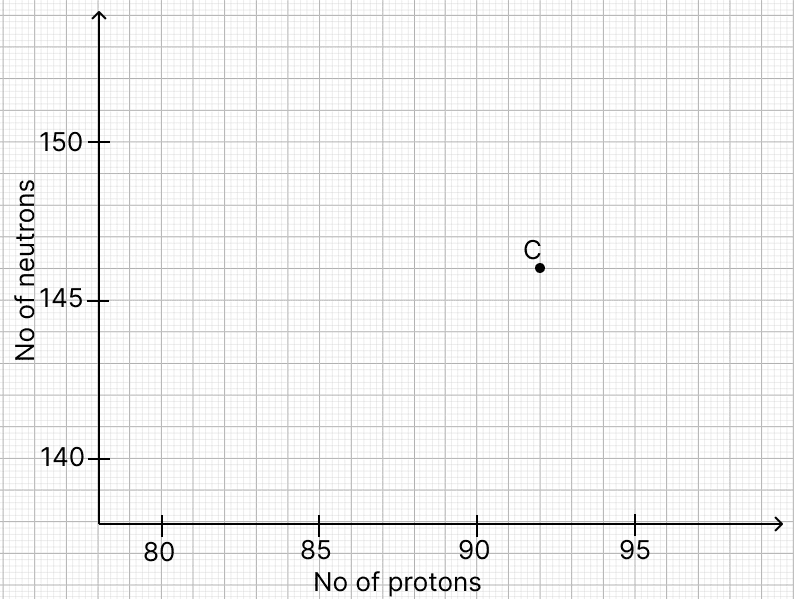
(a) Write the chemical symbol along with the mass number and atomic number for the element denoted as ‘C’.
(b) Plot on the graph,
i. The daughter element after the emission of beta radiation by the element ‘C’.
ii. The daughter element, as indicated in the previous answer, if it undergoes alpha decay.
Radioactivity
2 Likes
Answer
(a)
(b)
(i)
Here,
No. of protons of daughter element = atomic number = 93
No. of neutrons of daughter element = mass number - atomic number = 238 - 93 = 145
(ii)
Here,
No. of protons of daughter element = atomic number = 91
No. of neutrons of daughter element = mass number - atomic number = 234 - 91 = 143

Answered By
1 Like
Related Questions
The diagram below shows two parallel wires carrying current in the downward direction. The magnitude of the current in wire A is greater than in wire B. It is also observed that the wires exert force on each other.
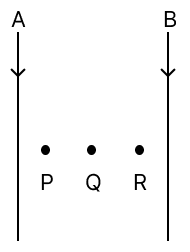
(a) State the direction of the force experienced by wire A.
(b) Name the law/laws used to come to the conclusion in (a).
(c) When a compass is placed in between the two wires, it does not remain along the direction of the magnetic field of wire A or wire B at one of the points P, Q, or R. State with a reason at which point you will observe this.
Shan is utilising a dynamometer to measure the force, expressed in newtons, on a bowstring. He records both the force and displacement of the bowstring and plots the data on a graph that shows the relationship between force in newtons and displacement in centimetres. Shan is interested in determining the highest point that the arrow reaches. He knows he can calculate the work done by finding the area under the graph.
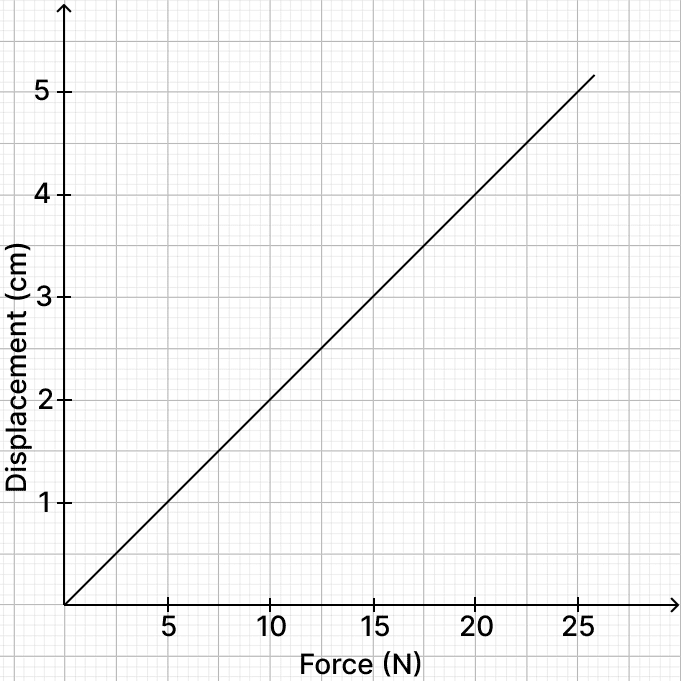
(a) What is the potential energy stored in the bow string, when the force is 25 N?
(b) If the mass of the arrow is 31.25 g, calculate the maximum velocity attained by the arrow.
(c) Calculate the maximum height reached by the arrow.
The adjacent diagram shows four solid plastic balls with wires fitted on a wooden base. A person shakes the wooden base to and fro (forward and backward) periodically. It is observed that even the balls start vibrating. It is also observed that all balls vibrate, but only one ball vibrates vigorously.
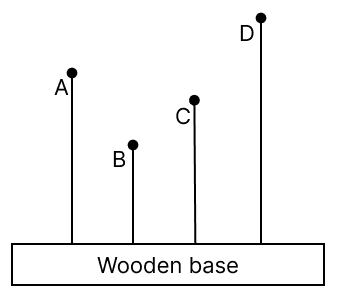
(a) Explain why only one ball vibrates vigorously.
(b) If fA, fB, fC, and fD are the natural frequencies of vibration of the wires, then arrange them in the increasing order of their frequencies and give reasons for the same.
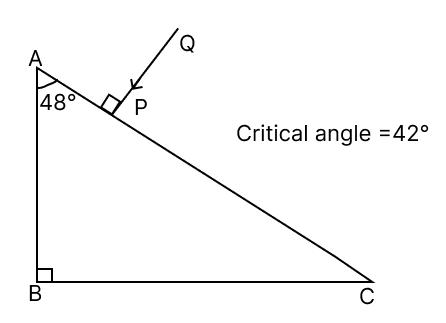
The above diagram shows a glass prism of a critical angle 42°.
(a) Redraw the diagram and complete the path of the light ray PQ till it emerges out of the prism.
(b) Also, calculate the net angle of deviation of the ray PQ when it emerges from the prism.