Physics
The inter-molecular forces in liquids are :
- as strong as in solids
- stronger than in solids
- weaker than in solids
- weaker than in gases
Matter
5 Likes
Answer
weaker than in solids
Reason —
Solids : Molecules are tightly packed, and intermolecular forces are strongest, giving them a fixed shape and volume.
Liquids : Molecules are less tightly packed compared to solids, so the intermolecular forces are weaker — allowing them to flow but still maintain a definite volume.
Gases : Molecules are far apart, and intermolecular forces are negligible — they can expand to fill any container.
Answered By
3 Likes
Related Questions
The molecules :
- in solid, liquid and gas, move freely anywhere.
- in a solid, move freely within its boundary.
- in a liquid, move within its boundary.
- in a gas, move only within its boundary.
Solids are :
- more dense
- less dense
- least dense
- highly compressible
The diagram below shows the arrangement of molecules in three states of matter.
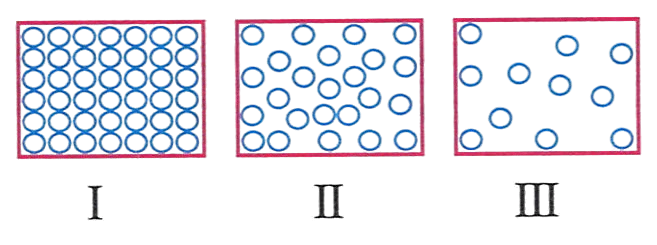
The arrangement of molecules in drinking water would look like.
- I
- II
- III
- all of these
Fill in the blanks :
(a) All the molecules of a substance are …………… .
(b) The inter-molecular spacing is …………… in solids …………… in liquids and …………… in gases.
(c) The molecular motion in liquid and gas is in …………… path.
(d) In a solid, the molecules …………… but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in …………… .
(f) A solid exerts pressure …………… .
(g) Gases are …………… dense.
(h) Solids are …………… rigid.