Chemistry
Name the anion present in each of the following compounds.
(a) Compound A when warmed with concentrated sulphuric acid gives a gas which fumes in moist air and which gives dense white fumes with ammonia.
(b) When barium chloride solution is added to a solution of compound B, a white precipitate insoluble in dilute hydrochloric acid is formed.
(c) The action of heat on the insoluble compound C produces a gas which turns lime water turbid.
(d) Compound D when warmed with dilute sulphuric acid gives a gas which turns acidified dichromate solution green.
Practical Chemistry
11 Likes
Answer
(a) Cl-
(b) SO42-
(c) CO32-
(d) SO32-
Answered By
5 Likes
Related Questions
Identify :
(a) The flame test with a salt P gave a brick red flame. What is the cation in P.
(b) pH of liquid R is 10. What kind of substance is R ?
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
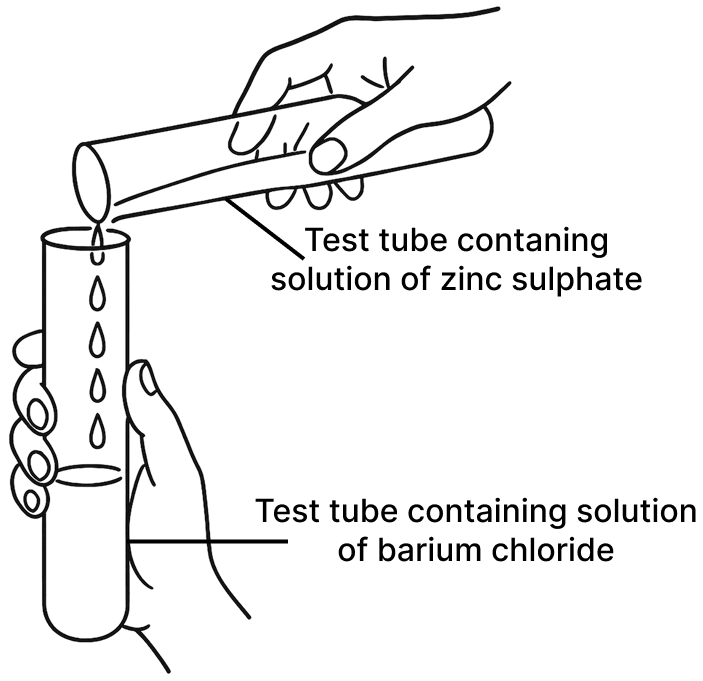
(b) Experiment 2
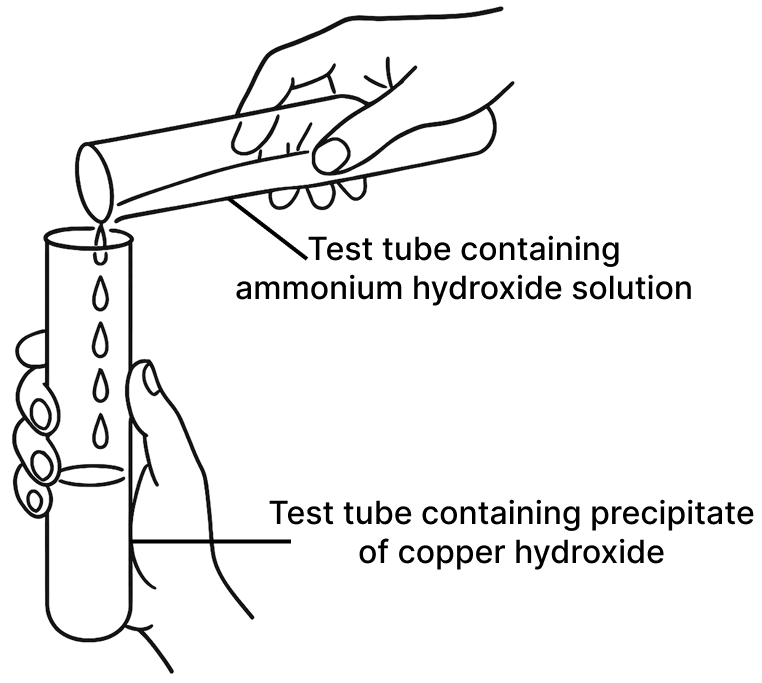
A given white crystalline salt was tested as follows :
(a) It dissolved in water and the resulting solution of the salt turned blue litmus red.
(b) Addition of barium chloride solution into this solution gave a white precipitate.
(c) A flame test on the salt gave a persistent golden-yellow colourisation.
What conclusion can be drawn for each observation?
(a) Sodium hydroxide solution is added to solution A. A white precipitate is formed which is insoluble in excess sodium hydroxide solution. Name the metal ion present in solution A.
(b) When ammonium hydroxide is added to solution B, a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide giving an inky blue solution. Name the cation present in solution B.
(c) When an ammonium salt is warmed with sodium hydroxide solution, ammonia gas is evolved. State three ways in which you could identify this gas.