Physics
A radioactive element P emits one α-particle and transforms to a new element Q. What will be the position of the element Q in the periodic table?
- One group to the left of P
- One group to the right of P
- Two groups to the right of P
- Two groups to the left of P
Radioactivity
2 Likes
Answer
Two groups to the left of P
Reason — The reaction is :
In periodic table elements are arranged in ascending order of atomic number and the atomic number of Q is 2 less than that of P. Hence, position of Q will 2 groups left of P.
Answered By
2 Likes
Related Questions
Assertion (A) : As the level of water in a tall measuring cylinder kept under running tap rises, the pitch of sound gradually increases.
Reason (R) : Frequency of sound is inversely proportional to the length of the water column.
- Both (A) and (R) are true and (R) is correct explanation of (A).
- Both (A) and (R) are true and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
In the given circuits Y and Z, the resistors, R1 and R2, are connected in :
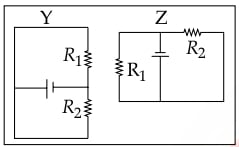
- series in both the circuits
- parallel in both the circuits
- parallel in Y and series in Z
- series in Y and parallel in Z
Each of the substances given below is supplied with same amount of heat. Which one will attain the highest temperature?
Substance Specific heat capacity (cal/g°C) Lead 0.031 Aluminium 0.21 Copper 0.095 Iron 0.115 - Aluminium
- Copper
- Iron
- Lead
The following figure shows a small bar magnet falling freely through a copper ring. For the observer at A, the direction of the induced current will be :
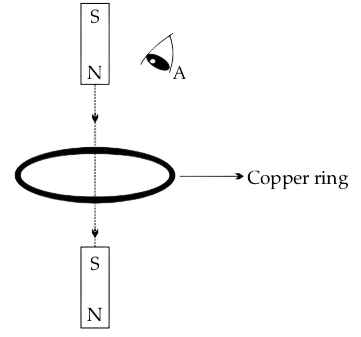
- clockwise when magnet is above and below the ring
- anticlockwise when magnet is above and below the ring
- anticlockwise when magnet is above the ring and clockwise when the magnet is below the ring
- clockwise when magnet is above the ring and anticlockwise when the magnet is below the ring