Physics
Study the diagram given below and answer the questions that follow :
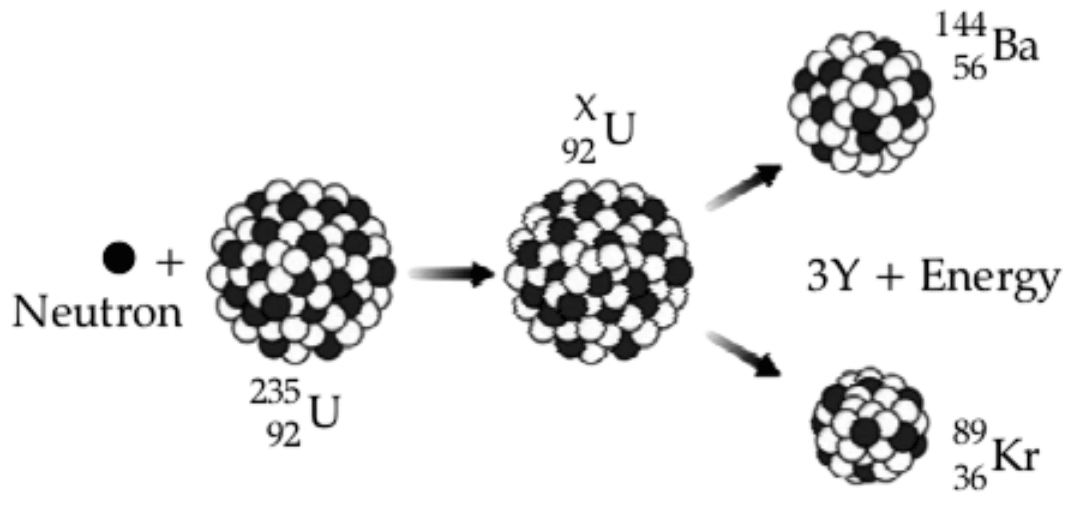
(a) Name the process depicted in the diagram.
(b) What is the value of X?
(c) Identify Y, the missing product of the reaction.
Radioactivity
2 Likes
Answer
(a) Nuclear fission.
(b) X = 236
(c) As, the mass number and atomic number is conserved in the reaction then
From conservation of mass number
Let, mass number of Y be y.
mass number of neutron + mass number of = 3 x mass number of Y + mass number of + mass number of
⇒ 236 = 3\text y + 233 \\[1em]
⇒ 3\text y = 236 - 233 = 3 \\[1em]
⇒ \text y = \dfrac{3}{3}=1
So, mass number of Y is 3.
From conservation of atomic number
Let, atomic number of Y be z.
atomic number of neutron + atomic number of = atomic number of Y + atomic number of + atomic number of
As mass number of Y is 1 and it's atomic number is zero which is a property of a neutron.
Hence, Y is a neutron.
Answered By
3 Likes
Related Questions
The diagrams given below show two sound boxes A and B with wires of same length (l) and tension (10 kgf) but different cross-sectional areas. Simultaneously, vibrating tuning forks of frequency 300 Hz are placed on the boxes A and B. The paper rider falls off in case of B but not in case of A.
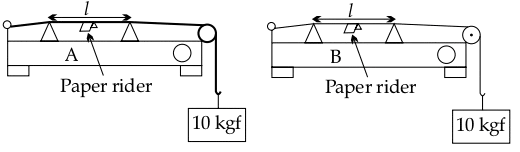
(a) Name and explain the phenomenon responsible for the falling off of the paper rider in B.
(b) The wire A resonates with a tuning fork of frequency ‘f’. Is ‘f’ greater than, less than or equal to 300 Hz? Justify your answer.
The diagram shows wiring in a meter room of a building.
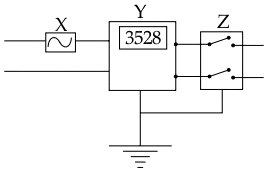
(a) What is the current rating of device X?
(b) What is the difference between the switch Z shown in the diagram and the switches you use to operate different appliances at home?
(c) What is the unit of the physical quantity displayed in Y?
Three identical bulbs B1, B2 and B3 each of power rating 18 W, 12 V are connected to a battery of 12 V.
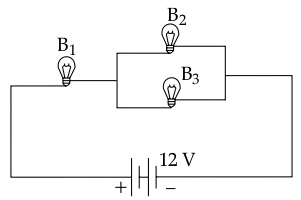
(a) Calculate :
- the resistance of each bulb
- the current drawn from the cell
(b) If the bulb B3 is removed from the circuit, then will the brightness of the bulb B1 increase, decrease or remain the same?
30 g of ice at 0°C is used to bring down the temperature of a certain mass of water at 70°C to 20°C. Find the mass of water.
[Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹ and specific latent heat of ice = 336 J g⁻¹]