Chemistry
When two organic compounds A and B react together in the presence of conc. H2SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B?
- 1.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-a-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-239x162.png)
- 2.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-b-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-252x123.png)
- 3.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-c-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-319x128.png)
- 4.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-d-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-181x97.png)
Organic Chemistry
4 Likes
Answer
- 3.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-c-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-319x128.png)
Reason — When an alcohol (–OH group) reacts with a carboxylic acid (–COOH group) in the presence of concentrated sulphuric acid, an ester is formed. Esters have a pleasant fruity smell. So, since A is an alcohol, B must be a carboxylic acid.
Example:
Answered By
1 Like
Related Questions
Which of the following chains of hydrocarbons undergoes two steps of reactions to become saturated?
- 1.
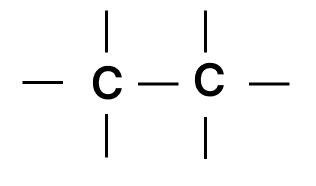
- 2.
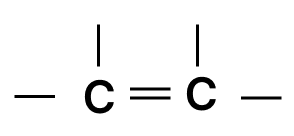
- 3.
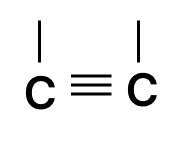
- 4.
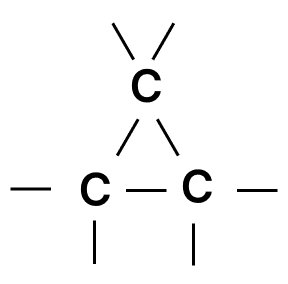
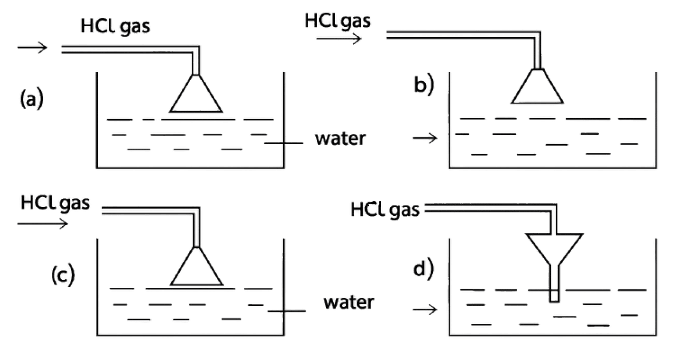
Given below are four different illustrations of preparing hydrochloric acid drawn by students. Which of these is the correct?
Given below are four covalent compounds.
(A) H2O (B) CCl4 (C) Cl2 (D) NH3
Which of the following represents the correct order when they are arranged in their increasing number of covalent bonds?
- B < D < A < C
- A < C < D < B
- C < D < A < B
- C < A < D < B
The electrolytic cell used for the electrolysis of molten lead bromide is made of Silica. Which of the following properties of silica that is the reason for it not having much significance in the process of electrolysis?
- Hard and strong
- Non-conductor of electricity
- Non-reactive
- Withstands high temperature