Chemistry
Given below are four different illustrations of preparing hydrochloric acid drawn by students. Which of these is the correct?
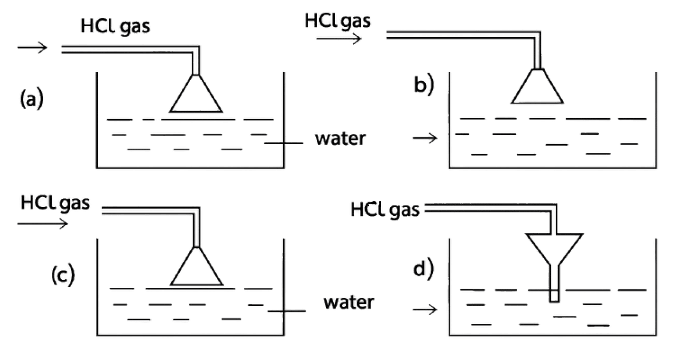
Hydrogen Chloride
2 Likes
Answer
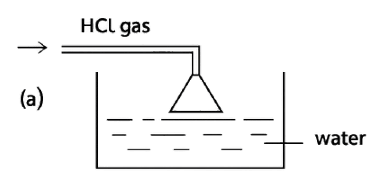
Reason — The inverted funnel is placed so that its rim just touches the water surface in the beaker. This setup is important for two main reasons:
- To prevent back-suction of water:
If the funnel mouth were dipped too deep into the water, a sudden cooling or stopping of gas flow could create a partial vacuum and suck water back into the gas supply tube. Keeping the rim just touching the water avoids this backward flow of water. - To increase the surface area for absorption:
The wide mouth of the funnel spreads the hydrogen chloride gas over a larger area of water. This helps the gas dissolve faster and more efficiently, since hydrochloric acid is prepared by dissolving hydrogen chloride gas in water.
Answered By
1 Like
Related Questions
When a compound was electrolysed using inert electrodes, the gas released at the anode made a glowing splinter rekindle. The electrolyte that will not produce such gas observation at the anode is:
- diluted solution of NaCl.
- concentrated solution of NaCl.
- diluted solution of copper sulphate.
- acidified water.
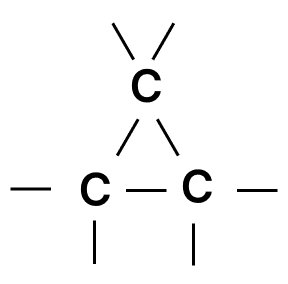
Which of the following chains of hydrocarbons undergoes two steps of reactions to become saturated?
- 1.
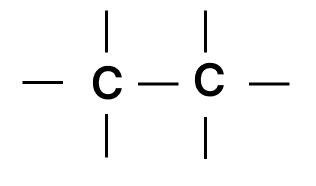
- 2.
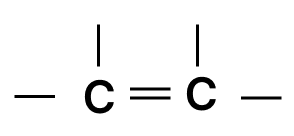
- 3.
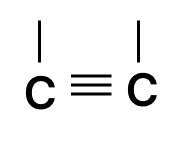
- 4.
When two organic compounds A and B react together in the presence of conc. H2SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B?
- 1.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-a-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-239x162.png)
- 2.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-b-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-252x123.png)
- 3.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-c-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-319x128.png)
- 4.
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-d-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-181x97.png)
Given below are four covalent compounds.
(A) H2O (B) CCl4 (C) Cl2 (D) NH3
Which of the following represents the correct order when they are arranged in their increasing number of covalent bonds?
- B < D < A < C
- A < C < D < B
- C < D < A < B
- C < A < D < B