Chemistry
When a compound was electrolysed using inert electrodes, the gas released at the anode made a glowing splinter rekindle. The electrolyte that will not produce such gas observation at the anode is:
- diluted solution of NaCl.
- concentrated solution of NaCl.
- diluted solution of copper sulphate.
- acidified water.
Electrolysis
3 Likes
Answer
concentrated solution of NaCl.
Reason — A gas released at the anode that caused a glowing splinter to rekindle is oxygen gas (O2). Concentrated solution of NaCl doesn't produce oxygen gas but it produces Chlorine which does not rekindle a glowing splinter.
Whereas, dilute HCl, dilute copper sulphate and acidified water produce oxygen gas when they are electrolysed using inert electrodes.
Answered By
1 Like
Related Questions
Which element forms a stable ion with the same electronic configuration as argon?
- Magnesium
- Fluorine
- Chlorine
- Sodium
The diagram below represents the molecule of an organic compound. What is the name of this compound?
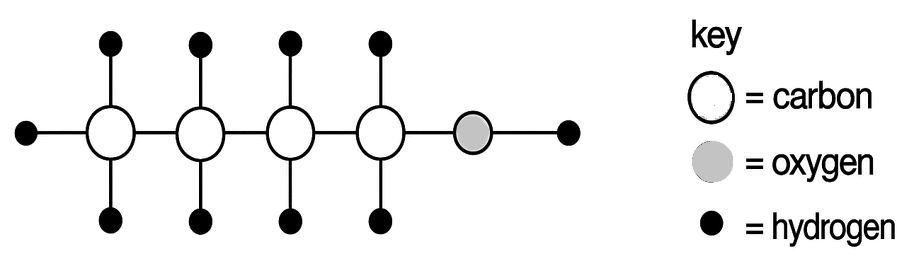
- Pentanol
- Butanol
- Butanoic acid
- Pentanoic acid
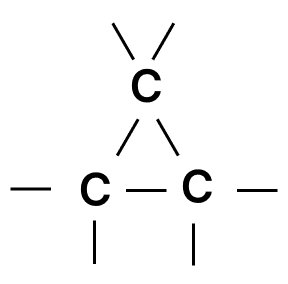
Which of the following chains of hydrocarbons undergoes two steps of reactions to become saturated?
- 1.
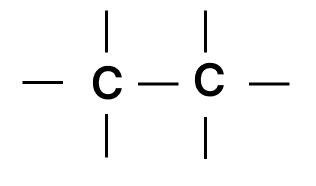
- 2.
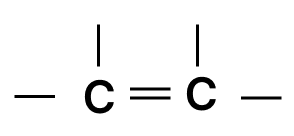
- 3.
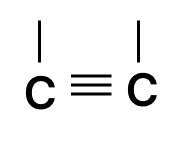
- 4.
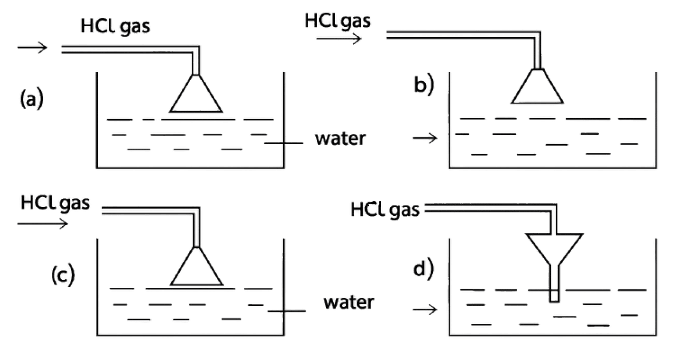
Given below are four different illustrations of preparing hydrochloric acid drawn by students. Which of these is the correct?