Chemistry
Which element forms a stable ion with the same electronic configuration as argon?
- Magnesium
- Fluorine
- Chlorine
- Sodium
Chemical Bonding
2 Likes
Answer
Chlorine
Reason — The electronic configuration of chlorine is 2, 8, 7. It has an electronic configuration with one electron less than that of the nearest noble gas, argon (2, 8, 8).
By acquiring an electronic configuration of argon, it will have one electron more than the number of protons in its nucleus, Hence, it will gain charge of -1.
Answered By
1 Like
Related Questions
The relative atomic mass of nitrogen is 14, and that of hydrogen is 1. This means that (i) …………… of nitrogen has the same mass as (ii) …………… of hydrogen.
(i) (ii) 1. An atom 28 molecules 2. An atom 7 molecules 3. A molecule 14 atoms 4. A molecule 7 atoms Which words correctly complete the gaps?
A student reacts copper turnings with cold dilute nitric acid in a test tube. He tests the gas given off with moist red and blue litmus paper. What is the name of the gas that evolved and what is the final colour of the litmus paper?
Gas Final colour of the litmus paper 1. NO No change in blue and red litmus paper 2. NO2 Blue litmus turns red and no change in red litmus 3. N2 No change in blue and red litmus paper 4. N2O No change in blue and red litmus paper The diagram below represents the molecule of an organic compound. What is the name of this compound?
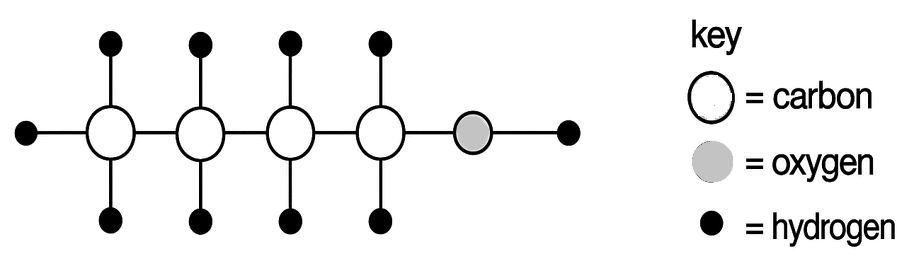
- Pentanol
- Butanol
- Butanoic acid
- Pentanoic acid
When a compound was electrolysed using inert electrodes, the gas released at the anode made a glowing splinter rekindle. The electrolyte that will not produce such gas observation at the anode is:
- diluted solution of NaCl.
- concentrated solution of NaCl.
- diluted solution of copper sulphate.
- acidified water.