Chemistry
The relative atomic mass of nitrogen is 14, and that of hydrogen is 1. This means that (i) …………… of nitrogen has the same mass as (ii) …………… of hydrogen.
| (i) | (ii) | |
|---|---|---|
| 1. | An atom | 28 molecules |
| 2. | An atom | 7 molecules |
| 3. | A molecule | 14 atoms |
| 4. | A molecule | 7 atoms |
Which words correctly complete the gaps?
Stoichiometry
5 Likes
Answer
An atom / 7 molecules
Reason — Atomic mass of Nitrogen is 14, atomic mass of hydrogen is 1.
7 molecules of hydrogen = 7 x H2 = 7 x 2 (H) = 7 x 2 = 14.
Hence, atomic mass of one atom of nitrogen is same as 7 molecules of Hydrogen.
Answered By
2 Likes
Related Questions
Element Y is in Group IIA of the Periodic Table. Y reacts with element Q to form an ionic compound. Which equation shows the process that takes place when Y forms ions?
- Y + 2e- ⟶ Y2+
- Y - 2e- ⟶ Y2-
- Y + 2e- ⟶ Y2-
- Y - 2e- ⟶ Y2+
The diagram below shows a circuit used to electrolyse aqueous sodium argento cyanide. Which arrow indicates the movement of the silver ions in the electrolyte and of the electrons in the external circuit?
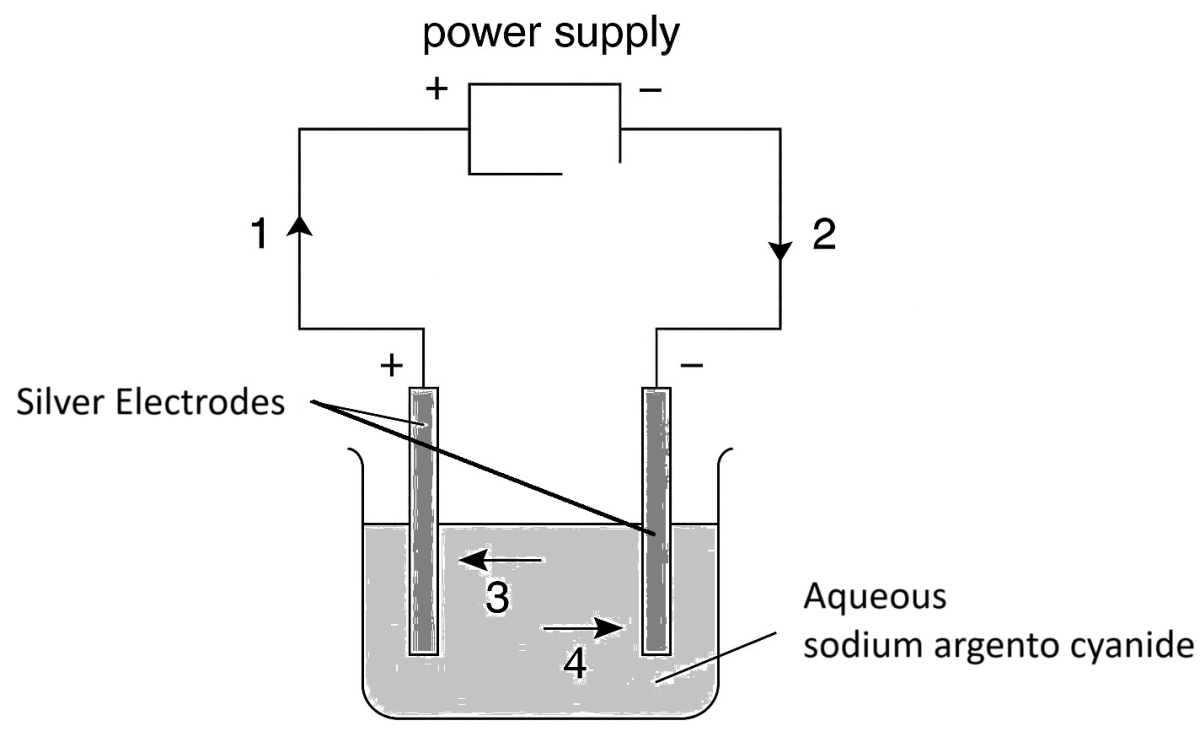
Silver ions Electrons 1. 3 1 2. 3 2 3. 2 4 4. 4 1 A student reacts copper turnings with cold dilute nitric acid in a test tube. He tests the gas given off with moist red and blue litmus paper. What is the name of the gas that evolved and what is the final colour of the litmus paper?
Gas Final colour of the litmus paper 1. NO No change in blue and red litmus paper 2. NO2 Blue litmus turns red and no change in red litmus 3. N2 No change in blue and red litmus paper 4. N2O No change in blue and red litmus paper Which element forms a stable ion with the same electronic configuration as argon?
- Magnesium
- Fluorine
- Chlorine
- Sodium