Chemistry
Element Y is in Group IIA of the Periodic Table. Y reacts with element Q to form an ionic compound. Which equation shows the process that takes place when Y forms ions?
- Y + 2e- ⟶ Y2+
- Y - 2e- ⟶ Y2-
- Y + 2e- ⟶ Y2-
- Y - 2e- ⟶ Y2+
Periodic Table
4 Likes
Answer
Y - 2e- ⟶ Y2+
Reason — Group IIA elements are alkaline earth metals with 2 electrons in their outermost shell. To achieve a stable noble gas configuration, they prefer to lose these 2 electrons rather than gain more forming a positive ion. Hence, the equation will be
Y - 2e- ⟶ Y2+
Answered By
2 Likes
Related Questions
Group number 1A
1IIA
2IIIA
13IVA
14VA
15VIA
16VIIA
17VIIIA
18Li D O J Ne A Mg E Si H K B C F G L With reference to the portion of the periodic table given above, identify the element having the largest atomic size:
- Li
- B
- K
- L
The picture given below shows an apparatus that a teacher used for demonstrating the properties of ionic substances. The teacher heats a sample of lead bromide in a crucible which contains two electrodes which are part of the circuit shown. The bulb does not light up. What is the best explanation for this?
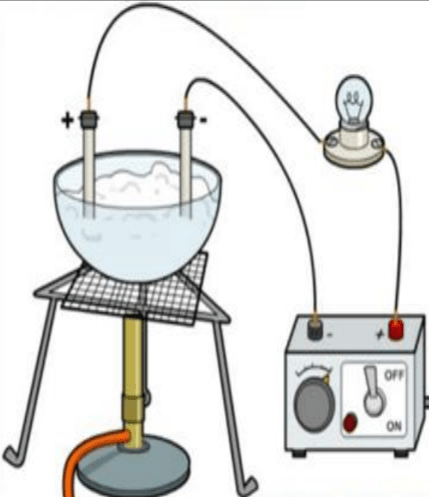
- The circuit is complete.
- Molten lead bromide does not conduct electricity.
- The sample of lead bromide was not heated up to the melting point by the teacher.
- The DC power supply was set up correctly.
The diagram below shows a circuit used to electrolyse aqueous sodium argento cyanide. Which arrow indicates the movement of the silver ions in the electrolyte and of the electrons in the external circuit?
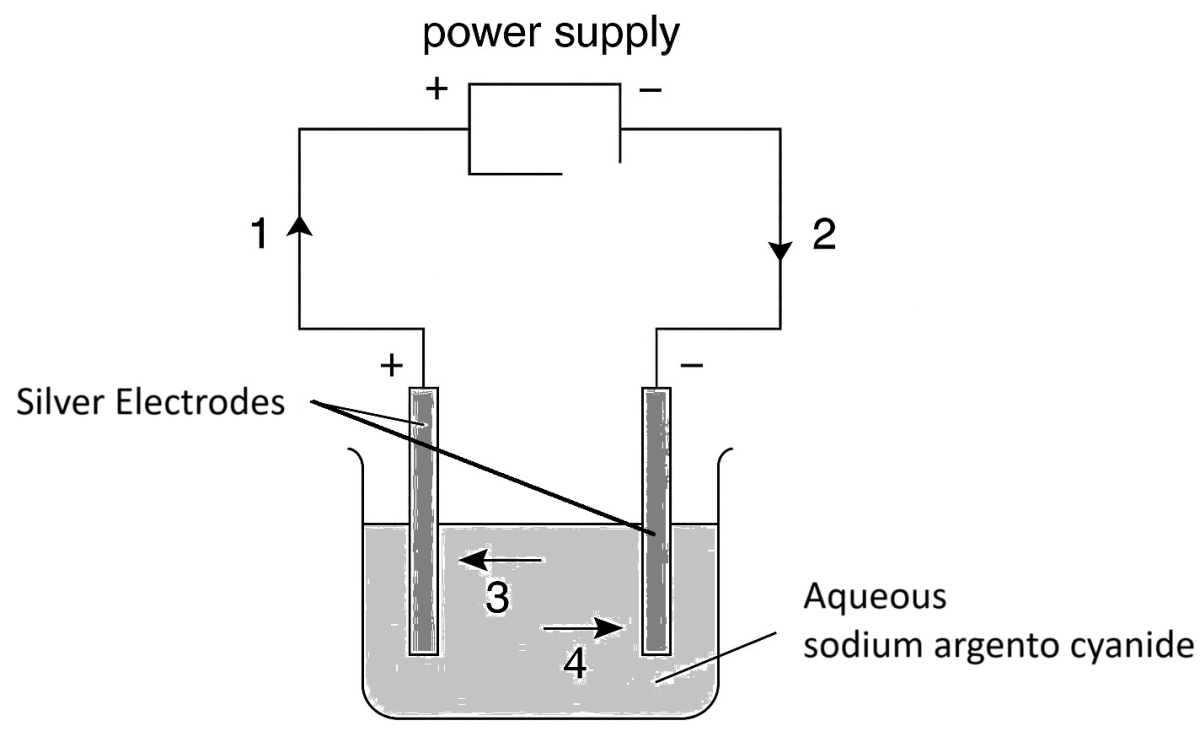
Silver ions Electrons 1. 3 1 2. 3 2 3. 2 4 4. 4 1 The relative atomic mass of nitrogen is 14, and that of hydrogen is 1. This means that (i) …………… of nitrogen has the same mass as (ii) …………… of hydrogen.
(i) (ii) 1. An atom 28 molecules 2. An atom 7 molecules 3. A molecule 14 atoms 4. A molecule 7 atoms Which words correctly complete the gaps?