Physics
Write true or false for each statement :
(a) The molecules of each substance are identical.
(b) The inter-molecular forces are effective at all distances between the two molecules.
(c) The molecules in a substance are in random motion.
(d) In a gas, the molecules can move anywhere in space.
(e) Liquids are less viscous than gases.
Matter
7 Likes
Answer
(a) False because different substances (e.g. air, water etc.) contain different types of molecules, so they are not identical
(b) False as intermolecular forces act only at very short distances (a few nanometers). At large distances, they become negligible.
(c) True since the particles of matter are not at rest, but they move randomly in all possible directions in a zig zag path.
(d) True as the inter-molecular forces are very weak in a gas which makes the molecules wide apart and hence, they can move freely in space.
(e) False because liquids are generally more viscous than gases because their molecules are closer together, leading to more friction between different layers.
Answered By
3 Likes
Related Questions
The diagram below shows the arrangement of molecules in three states of matter.
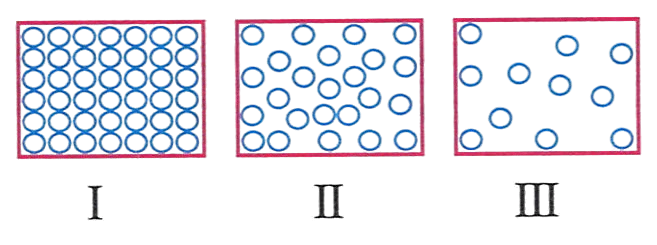
The arrangement of molecules in drinking water would look like.
- I
- II
- III
- all of these
Fill in the blanks :
(a) All the molecules of a substance are …………… .
(b) The inter-molecular spacing is …………… in solids …………… in liquids and …………… in gases.
(c) The molecular motion in liquid and gas is in …………… path.
(d) In a solid, the molecules …………… but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in …………… .
(f) A solid exerts pressure …………… .
(g) Gases are …………… dense.
(h) Solids are …………… rigid.
Match the following columns :
Column A Column B (a) A molecule is composed of (i) does not exist free in nature. (b) Ice, water and water vapour (ii) can vibrate only up to about 10-10 m from their mean positions. (c) An atom (iii) atoms. (d) Gases (iv) are the three states of water. (e) The molecules of a solid (v) occupy space Define matter. What is its composition?