Physics
85 g of water at 30°C is cooled to 5°C by adding certain mass of ice. Find the mass of ice required.
[Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹ , Specific latent heat of fusion = 336 J g⁻¹]
Calorimetry
4 Likes
Answer
Given,
For Water
Mass of water (mw) = 85 g
Initial temperature (Ti) = 30°C
Final temperature (Tf) = 5°C
Specific heat capacity of water (cw) = 4.2 J g⁻¹ °C⁻¹
For ice
Initial temperature (Ti) = 0°C
Final temperature (Tf) = 5°C
Specific latent heat of fusion (Li) = 336 J g⁻¹
Let,
Mass of ice = (mi)
Now,
Heat lost by water = mwcw△t
= 85 × 4.2 × (30 - 5)
= 85 × 4.2 × 25 = 8925 J
Heat gained by ice = miLi + micw△t
= mi × 336 + mi × 4.2 × (5-0)
= 336mi + mi × 4.2 × 5
= 336mi + 21mi = 357mi
By principle of calorimetry,
Heat lost by water = Heat gained by ice
⟹ 8925 = 357 mi
⟹ mi = = 25 g
So, the mass of required ice is 25 g.
Answered By
2 Likes
Related Questions
Below is the diagram of a transformer :
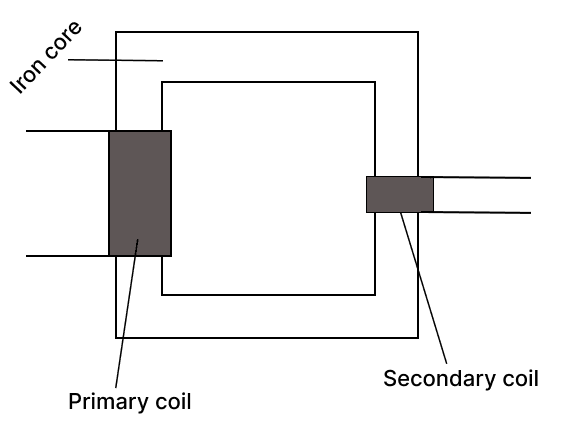
(a) Identify the type of transformer.
(b) In this type of transformer which of the wire is thicker, the primary or the secondary? Give a reason.
Study the diagram :
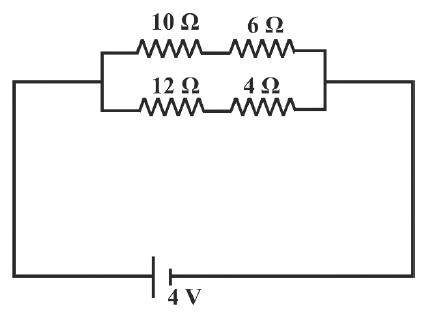
(a) Calculate the total resistance of the circuit.
(b) Calculate the current drawn from the cell.
(c) State whether the current through 10 Ω resistor is greater than, less than or equal to the current through the 12 Ω resistor.
(a) Why does it become pleasantly warm when the lakes start freezing?
(b) Water freezes to form ice. What change would you expect in the average kinetic energy of the molecules?
(c) Which will contain more heat energy 1 g of ice at 0°C or 1 g water at 0°C ?
(a) State one factor that affects the magnitude of induced current in an AC generator.
(b) Given below is a circuit to study the magnetic effect of electric current. ABCD is a cardboard kept perpendicular to the conductor XY. A magnetic compass is placed at the point P of the cardboard. P1 and P2 are the positions of the magnetic compass, before and after passing a current through XY respectively.
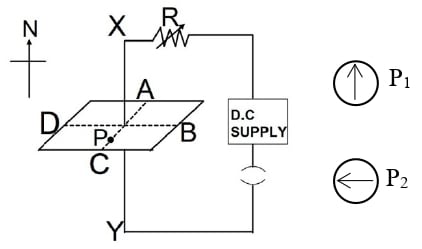
- Name the rule that is used to predict the direction of deflection of the magnetic compass.
- State the direction of current in the conductor (X to Y or Y to X) when the circuit is complete.
- If resistance R is increased, then what will be the effect on the magnetic lines of force around the conductor?