Physics
The figure given below shows a radioactive nucleus A composed of 84 protons and 128 neutrons kept in a thick lead walled container. The emitted particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by X. The nucleus A emits a particle which deflects to the left and is transformed into nucleus B. Nucleus B further emits a particle which deflects towards right and transforms into nucleus C.
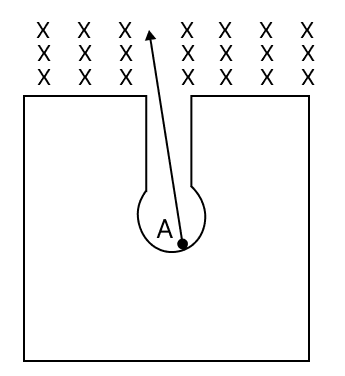
(a) Name the radiations emitted by nucleus A and B.
(b) Name the law used to identify the radiations.
(c) What is the composition of nucleus C?
Radioactivity
5 Likes
Answer
(a) Nucleus A emits alpha (α) radiation and nucleus B emits beta (β) radiation.
As particles emitted by nucleus A deflect to left hence, they are alpha (α) particles and nucleus B emission deflects to the right hence, they are beta (β) particles.
(b) Fleming's left hand rule.
(c) Nucleus C will have 83 protons and 125 neutrons.
Explanation:
Given,
Before any decay :
Number of protons in A = 84 = Atomic number of A
Number of neutrons in A = 128
Mass number = 84 + 128 = 212
After alpha decay (A → B) :
Loss of protons = 2
Loss of neutrons = 2
New atomic number = 84 – 2 = 82
New mass number = 212 – 4 = 208
After beta decay (B → C) :
As in beta decay, a neutron converts into a proton so that Atomic number increases by 1 and mass number remains the same.
New atomic number = 82 + 1 = 83
Mass number = 208
For C
Number of protons = atomic number of C = 83
Number of neutrons = Mass number - number of proton = 208 - 83 = 125
Answered By
2 Likes
Related Questions
State condition in each case for the magnitude of force on a current carrying conductor placed in a magnetic field to be (a) zero, (b) maximum
Arrange the α, ẞ and γ radiations in ascending order of their (i) ionising power, and (ii) penetrating power.
Jatin puts a pencil into a glass container having water and is surprised to see the pencil in a different state.
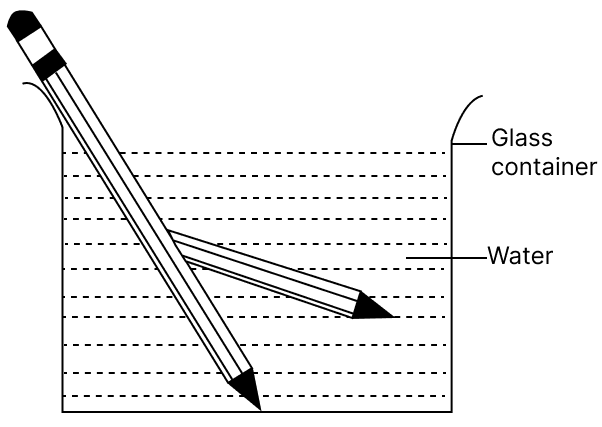
(a) What change is observed in the appearance of the pencil?
(b) What is the reason for this change?
(c) Classify the following as a real or virtual image:
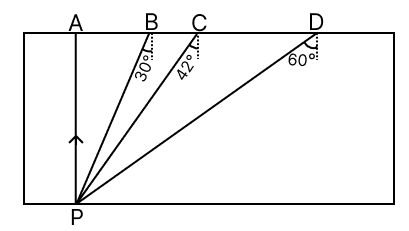
The image of a candle flame formed on a screen by a convex lens.The adjacent diagram represents a glass slab of refractive index 1.5. If PA, PB, PC and PD represent the rays of light from a point P at the bottom of the block, draw the approximate directions of these rays as they emerge out of the glass slab. (sin 42° = 2/3)